Question: D30 El jefe (the chief) decided to do something to improve the low conversion (XA = 0.95) of our first order solid-catalyzed liquid phase reaction.
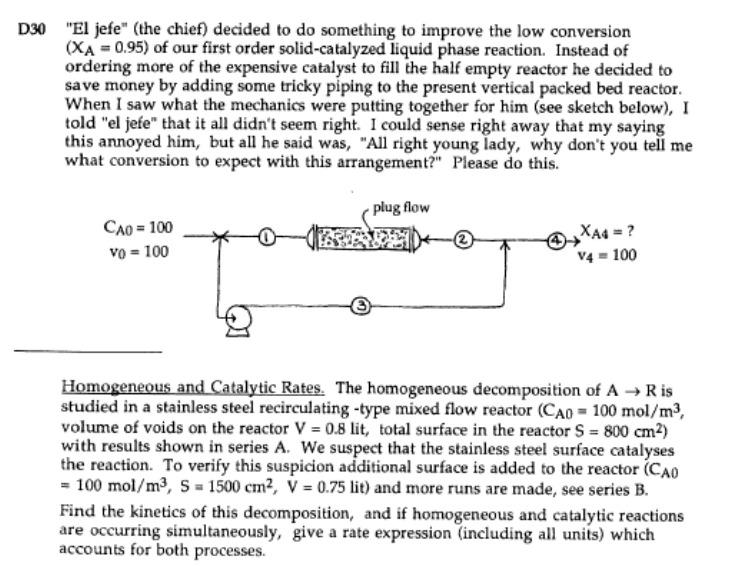
D30 "El jefe" (the chief) decided to do something to improve the low conversion (XA = 0.95) of our first order solid-catalyzed liquid phase reaction. Instead of ordering more of the expensive catalyst to fill the half empty reactor he decided to save money by adding some tricky piping to the present vertical packed bed reactor. When I saw what the mechanics were putting together for him (see sketch below), I told "el jefe" that it all didn't seem right. I could sense right away that my saying this annoyed him, but all he said was, "All right young lady, why don't you tell me what conversion to expect with this arrangement?" Please do this. plug flow CAO = 100 Vo = 100 XA4 = ? V4 = 100 Homogeneous and Catalytic Rates. The homogeneous decomposition of AR is studied in a stainless steel recirculating -type mixed flow reactor (CAO - 100 mol/m3, volume of voids on the reactor V = 0.8 lit, total surface in the reactor S = 800 cm2) with results shown in series A. We suspect that the stainless steel surface catalyses the reaction. To verify this suspicion additional surface is added to the reactor (CAO = 100 mol/m3, S = 1500 cm2, V = 0.75 lit) and more runs are made, see series B. Find the kinetics of this decomposition, and if homogeneous and catalytic reactions are occurring simultaneously, give a rate expression (including all units) which accounts for both processes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


