Question: Dashboard My courses 20201 (1) - General (1) - Question 9 Not yet answered Given the following values of electronegativity (EN) Mg=1.31 5=2.58 0=344 Then
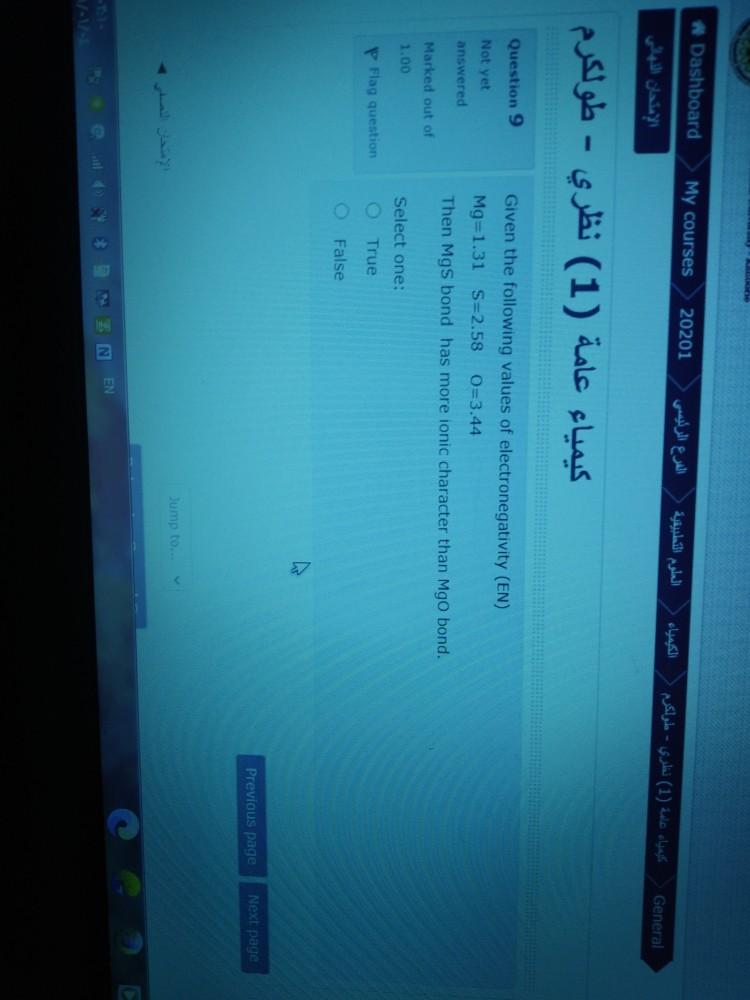
Dashboard My courses 20201 (1) - General (1) - Question 9 Not yet answered Given the following values of electronegativity (EN) Mg=1.31 5=2.58 0=344 Then Mgs bond has more ionic character than Mgo bond. Marked out of 10 Select one: True P Flag question False Previous page Next page Jump to. - EN EN
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


