Question: Data Analysis and Calculations PART B Standardizing Sodium Hydroxide TABLE 3.1 Data to Determine Molarity of NaOH Solution NaOH (ml) V. NaOH (IL) used 0.57

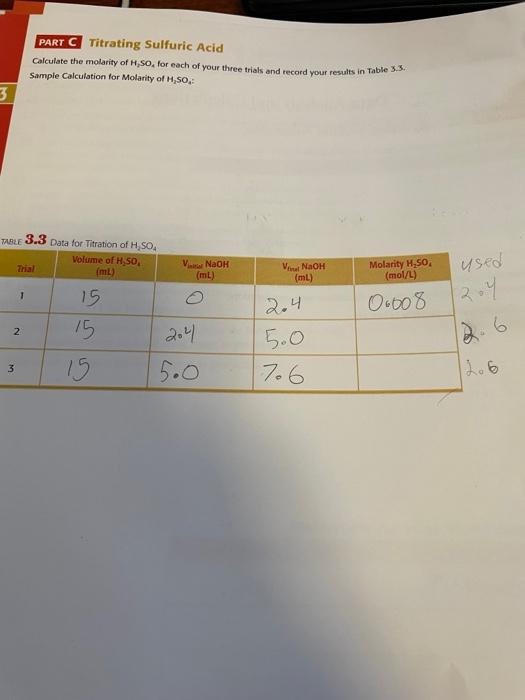
Data Analysis and Calculations PART B Standardizing Sodium Hydroxide TABLE 3.1 Data to Determine Molarity of NaOH Solution NaOH (ml) V. NaOH (IL) used 0.57 25 20mL Trial Mass of KHPD 1 2 468 0.51 10.5 7.4 204 3 0.0 Calculate the molarity of NaOH for each of your three trials in Table 3.2. Use the average of these values for your subsequent work. Check your molarity calculation with your instructor. Sample Calculation for Molarity of NaOH: TABLE 3.2 Results from Calculations of the Molarity of NaOH Trial Moles of KHP Moles of NaOH Molarity of NaOH 0.60279 0.2 2 2 3 Average NA NA PART C Titrating Sulfuric Acid Calculate the molarity of H.So, for each of your three trials and record your results in Table 3.5. Sample Calculation for Molarity of H,50, 3 TABLE 3.3 Data for Titration of H SO Volume of H,SO (ml) Var Nao (mL) Minat NaOH (ml) Molarity HSO (mol/L) used 1 15 0-008 2 204 15 15 2.4 5.0 7.6 3 5.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


