Question: The drawing below shows a mixture of molecules: carbon nitrogen oxygen key O hydrogen O sulfur Ochlorine Suppose the following chemical reaction can take
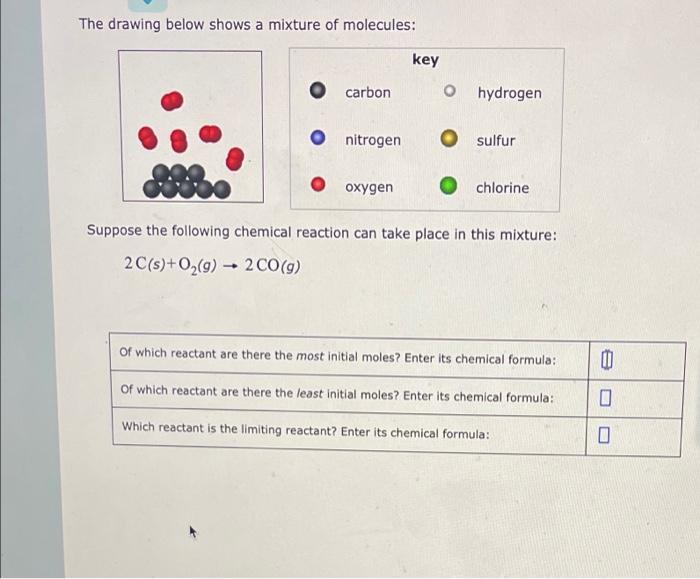

The drawing below shows a mixture of molecules: carbon nitrogen oxygen key O hydrogen O sulfur Ochlorine Suppose the following chemical reaction can take place in this mixture: 2 C(s) + O(g) 2 CO(g) - Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula: 0 0 Suppose 10.0 g of Compound A and 5.5 g of Compound B are consumed in a reaction that produces only one product, Compound C. Calculate the theoretical yield of C. Round your answer to the nearest 0.1 g. Suppose 9.5 g of C are actually isolated at the end of the reaction. What is the percent yield of Compound C? 4 kound your answer to the nearest whole percent. 0 8
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
There are 8 black balls Carbon atoms and 5 groups of red balls each group containing 2 red balls rep... View full answer

Get step-by-step solutions from verified subject matter experts


