Question: Data Quantity Object 1 Object 2 Mass of Object (g) 2.94 N 0 . 41 1 Mass of Empty Cup (g) | 0. 0316K. Q
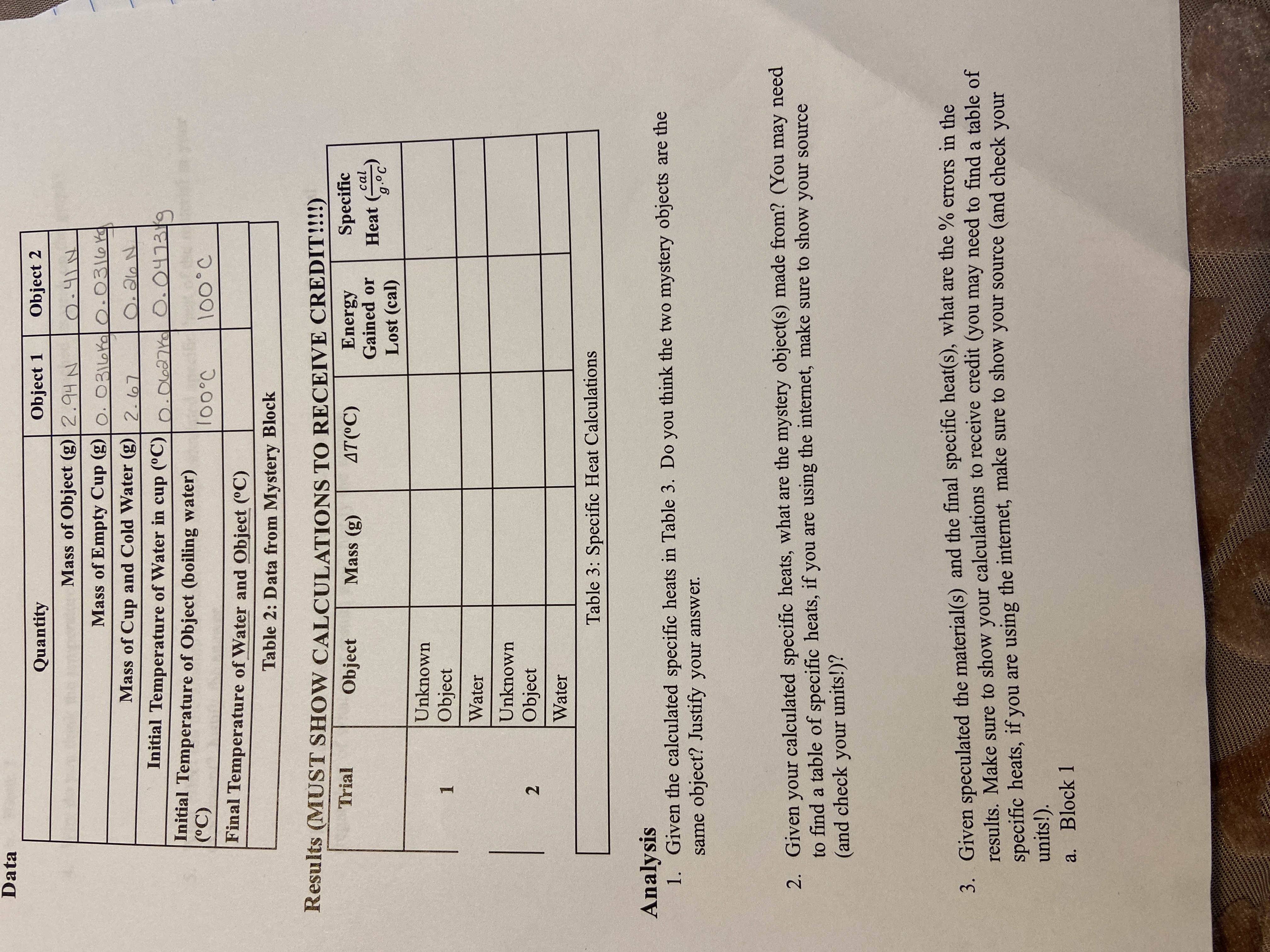
Data Quantity Object 1 Object 2 Mass of Object (g) 2.94 N 0 . 41 1 Mass of Empty Cup (g) | 0. 0316K. Q . 0316K Mass of Cup and Cold Water (g) 2. 67 O . 26 N Initial Temperature of Water in cup (C) 0 . 0627 KQ 0. 0473 / 9 Initial Temperature of Object (boiling water) (C) 100 .C 100.C Final Temperature of Water and Object (C) Table 2: Data from Mystery Block Results (MUST SHOW CALCULATIONS TO RECEIVE CREDIT!!) Trial Object Mass (g) AT(C) Energy Specific Gained or Heat (or Lost (cal) Unknown 1 Object Water Unknown Object Water Table 3: Specific Heat Calculations Analysis 1. Given the calculated specific heats in Table 3. Do you think the two mystery objects are the same object? Justify your answer. 2. Given your calculated specific heats, what are the mystery object(s) made from? (You may need to find a table of specific heats, if you are using the internet, make sure to show your source (and check your units!)? 3. Given speculated the material(s) and the final specific heat(s), what are the % errors in the results. Make sure to show your calculations to receive credit (you may need to find a table of specific heats, if you are using the internet, make sure to show your source (and check your units!). a. Block 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


