Question: DE - 3.57 X10- J DE 1.60 *10-19 = 2. 23 Mev 10. The mass of a helium-4 nucleus is 4.0026 u. The mass of
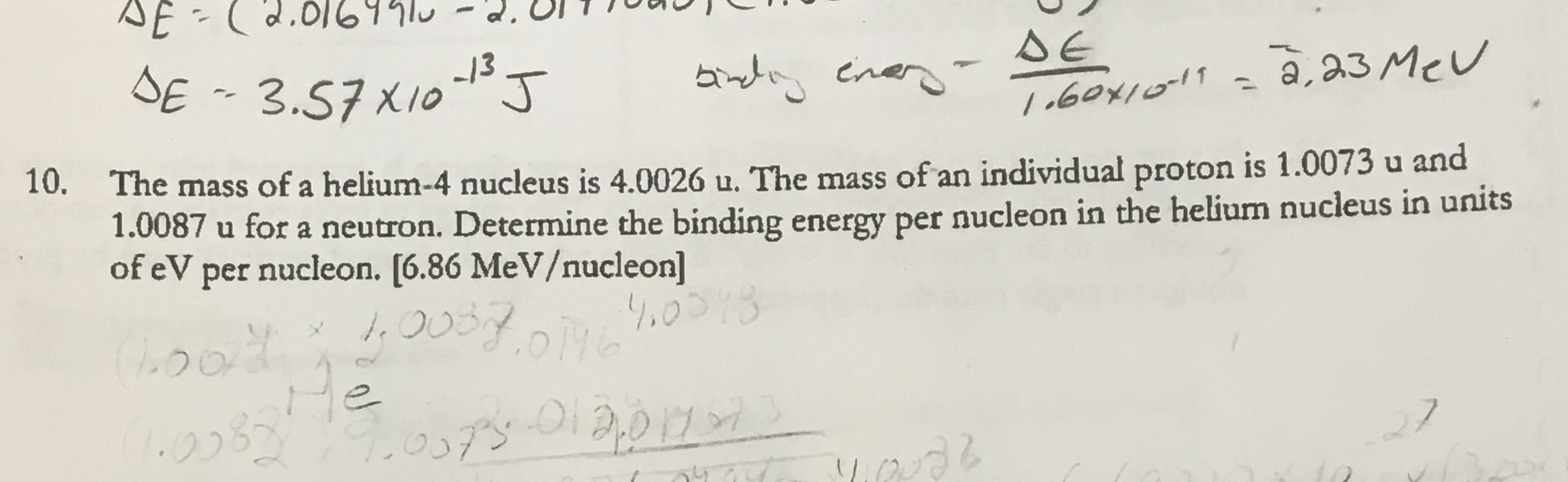
DE - 3.57 X10- J DE 1.60 *10-19 = 2. 23 Mev 10. The mass of a helium-4 nucleus is 4.0026 u. The mass of an individual proton is 1.0073 u and 1.0087 u for a neutron. Determine the binding energy per nucleon in the helium nucleus in units of eV per nucleon. [6.86 MeVucleon] x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


