Question: -de -TA dt Using PSSH, derive the rate law for using the following reaction mechanisms: A +BAB (k1= SRR for forward reaction; k2=SRR for backward
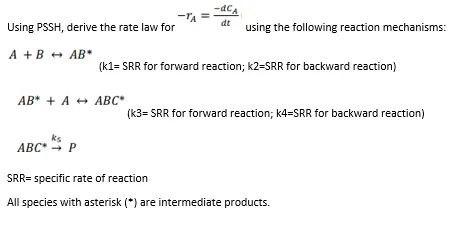
-de -TA dt Using PSSH, derive the rate law for using the following reaction mechanisms: A +BAB (k1= SRR for forward reaction; k2=SRR for backward reaction) AB* + A + ABC * A (k3= SRR for forward reaction; k4=SRR for backward reaction) ks ABC - P SRR= specific rate of reaction All species with asterisk (*) are intermediate products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


