Question: Dear Chegg Genius please help :) Mass data for preparation of alum 0.00 1.18 Mass of weighing paper (9) Mass of weighing paper plus aluminum
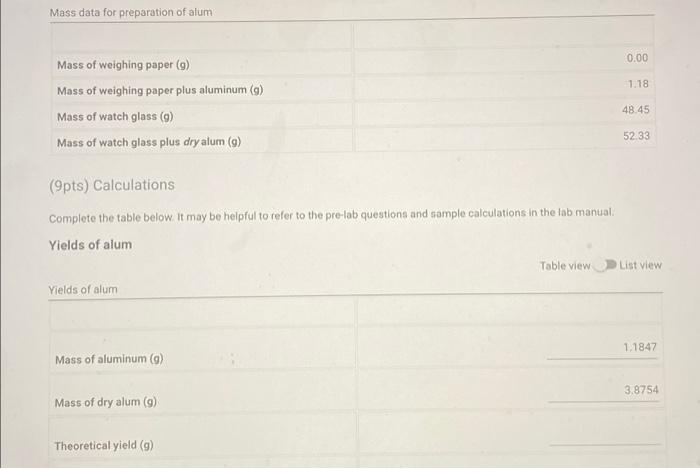
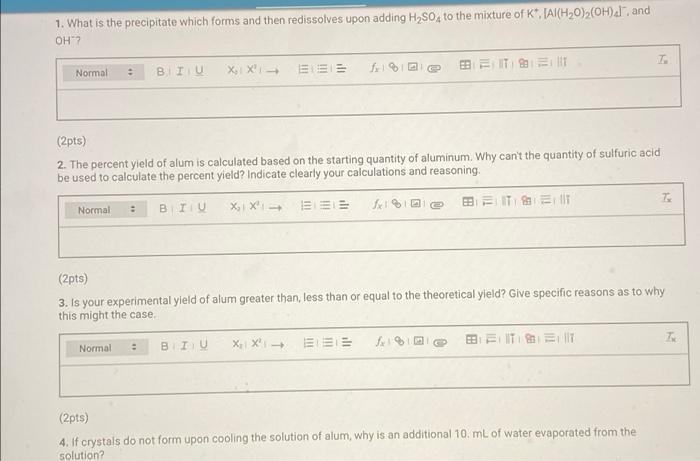
Mass data for preparation of alum 0.00 1.18 Mass of weighing paper (9) Mass of weighing paper plus aluminum (a) Mass of watch glass (9) Mass of watch glass plus dry alum (9) 48.45 52.33 (9pts) Calculations Complete the table below. It may be helpful to refer to the pre-lab questions and sample calculations in the lab manual Yields of alum Table view List view Yields of alum 1.1847 Mass of aluminum (9) 3.8754 Mass of dry alum (9) Theoretical yield (9) (8pts) Sample Calculations (Apts) Balanced chemical equation for conversion of Al(s) to KAl(SO4)2 12H2O(s) in aqueous solution T Normal BIU BIT SIT XX | 0 4 (2pts) For full credit, upload an image of your calculation for theoretical yield Browse your files to upload or Drag and Drop Mix attachments: 51 Max Size: 20.00MB cach (2pts nnenfun calentation for Dercent vield 1. What is the precipitate which forms and then redissolves upon adding H2SO, to the mixture of K", [Al(H20)(OH), and OH"? Normal BIU X X 123 (2pts) 2. The percent yield of alum is calculated based on the starting quantity of aluminum. Why can't the quantity of sulfuric acid be used to calculate the percent yield? Indicate clearly your calculations and reasoning Normal BIU XXE BEIT MIT T. (2pts) 3. Is your experimental yield of alum greater than, less than or equal to the theoretical yleld? Give specific reasons as to why this might the case IT Normal T X1 X1 (2pts) 4. If crystals do not form upon cooling the solution of alum, why is an additional 10 mL of water evaporated from the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


