Question: Decide whether each chemical reaction in the table below is an oxidation-reduction (redox) reaction. If the reaction is an of the reducing agent and the
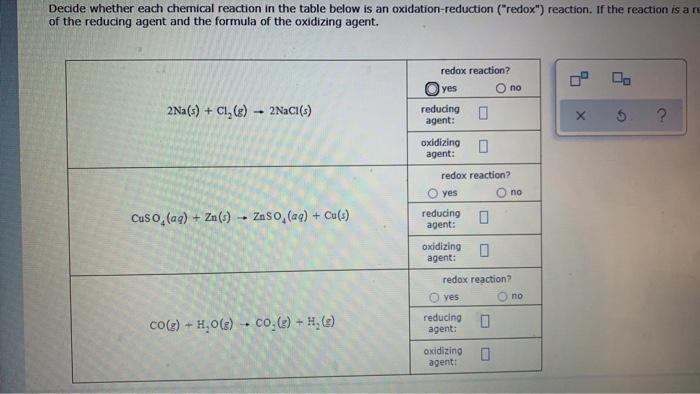
Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is an of the reducing agent and the formula of the oxidizing agent. redox reaction? yes reducing agent: 2Na(s) + Cl,CE) 2NaC1(5) ? oxidizing agent: redox reaction? O yes no reducing agent: Cuso (aq) + Zn () - Zn50 (aq) + Cu(s) oxidizing agent: redox reaction? ves no CO(e) + H2O() - CO.C) - H, (2) reducing agent: oxidizing agent
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


