Question: Decide whether these proposed Lewis structures are reasonable. E= . proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong
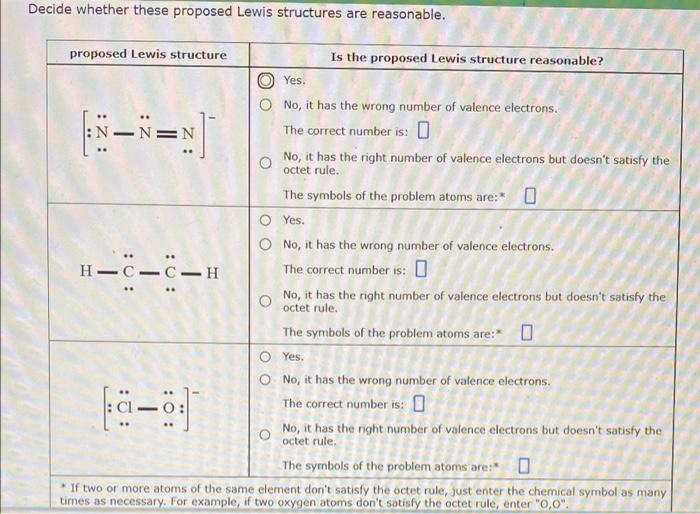
Decide whether these proposed Lewis structures are reasonable. E= . proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. N The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * O Yes. O No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: 0 O Yes. O No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: 0 * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0" H--C-H : o a-o o
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


