Question: Deduce the structure that fits the given data. - Molecular formula: C5H10O - Proton decoupled 13C NMR data: 14,17,30,45,207ppm - 1H NMR spectral data: 0.9(t,3H),1.60(m,5H),2.10(s,3H),2.40(t,2H).
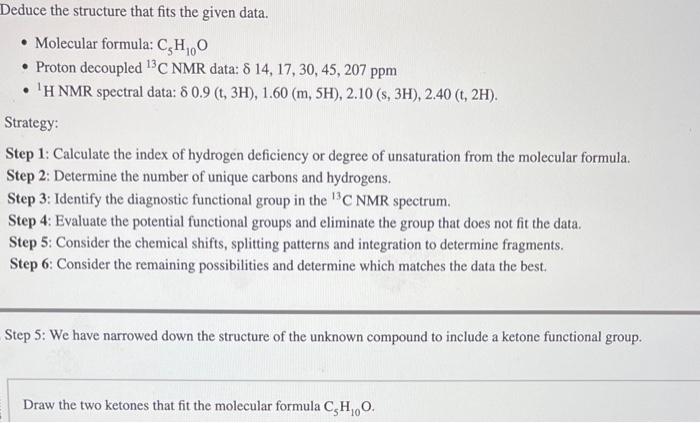
Deduce the structure that fits the given data. - Molecular formula: C5H10O - Proton decoupled 13C NMR data: 14,17,30,45,207ppm - 1H NMR spectral data: 0.9(t,3H),1.60(m,5H),2.10(s,3H),2.40(t,2H). Strategy: Step 1: Calculate the index of hydrogen deficiency or degree of unsaturation from the molecular formula. Step 2: Determine the number of unique carbons and hydrogens. Step 3: Identify the diagnostic functional group in the 13C NMR spectrum. Step 4: Evaluate the potential functional groups and eliminate the group that does not fit the data. Step 5: Consider the chemical shifts, splitting patterns and integration to determine fragments. Step 6: Consider the remaining possibilities and determine which matches the data the best. Step 5: We have narrowed down the structure of the unknown compound to include a ketone functional group. Draw the two ketones that fit the molecular formula C5H10O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


