Question: Define cyclic voltammetry. Label the following numbers (1, 2, 3 and 4) given below in cyclic voltammogram. ipa Current/A Potential / V lipc 1. 2.
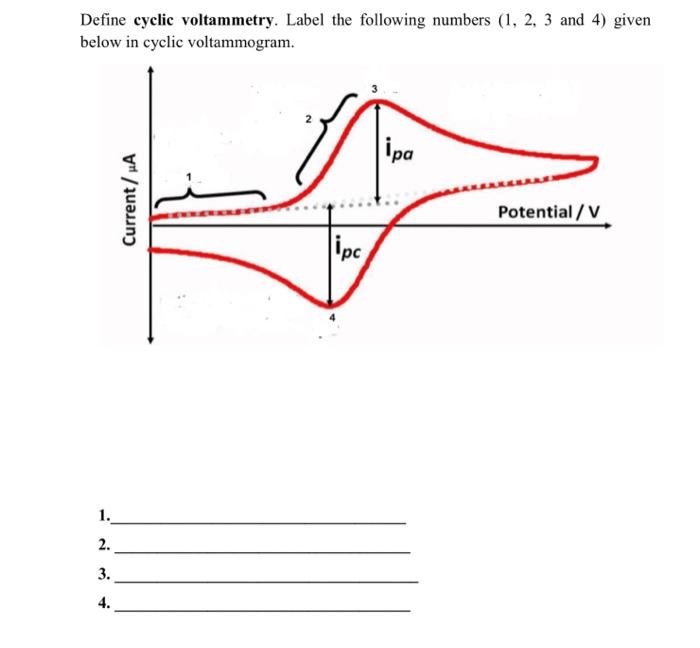


Define cyclic voltammetry. Label the following numbers (1, 2, 3 and 4) given below in cyclic voltammogram. ipa Current/A Potential / V lipc 1. 2. 3. 4. Use diagrams to distinguish between reversible, quasi reversible and irreversible redox reactions in cyclic voltammetry. Reversible redox reaction Quasi reversible redox reaction Irreversible redox reaction c)(1.5 points) Peak current of ferrocene in a cyclic voltammogram is 3.3uA at a scan rate of 50mV/s. The electrode area is 0.45cm- and the diffusion coefficient of ferrocene is 9x106cm/s. Considering n=1, calculate the concentration of ferrocene in solution using the following form of Randels-Sevcik equation? Ip=2.69x10n32AD12 Cv 1/2 where, Ip is peak current in Amperes. Constant 2.69 x10 in C 3/23-12mol! nis number of electrons having no unit A is area of electrode in m Dis diffusion coefficient in m/s Cis concentration in mol/m v is scan rate in V/s. Also show units with calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


