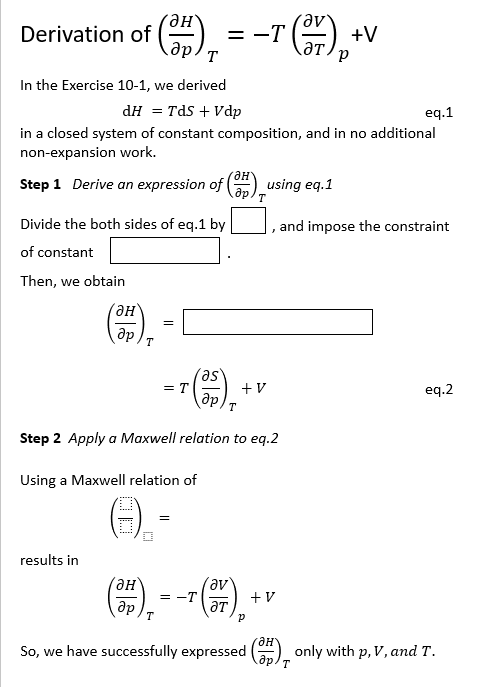
Question: Derivation of ( d e l H d e l p ) T = - T ( d e l V d e l T
Derivation of
In the Exercise we derived
in a closed system of constant composition, and in no additional
nonexpansion work.
Step Derive an expression of using eq
Divide the both sides of eq by and impose the constraint
of constant
Then, we obtain
Step Apply a Maxwell relation to eq
Using a Maxwell relation of
results in
So we have successfully expressed only with and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


