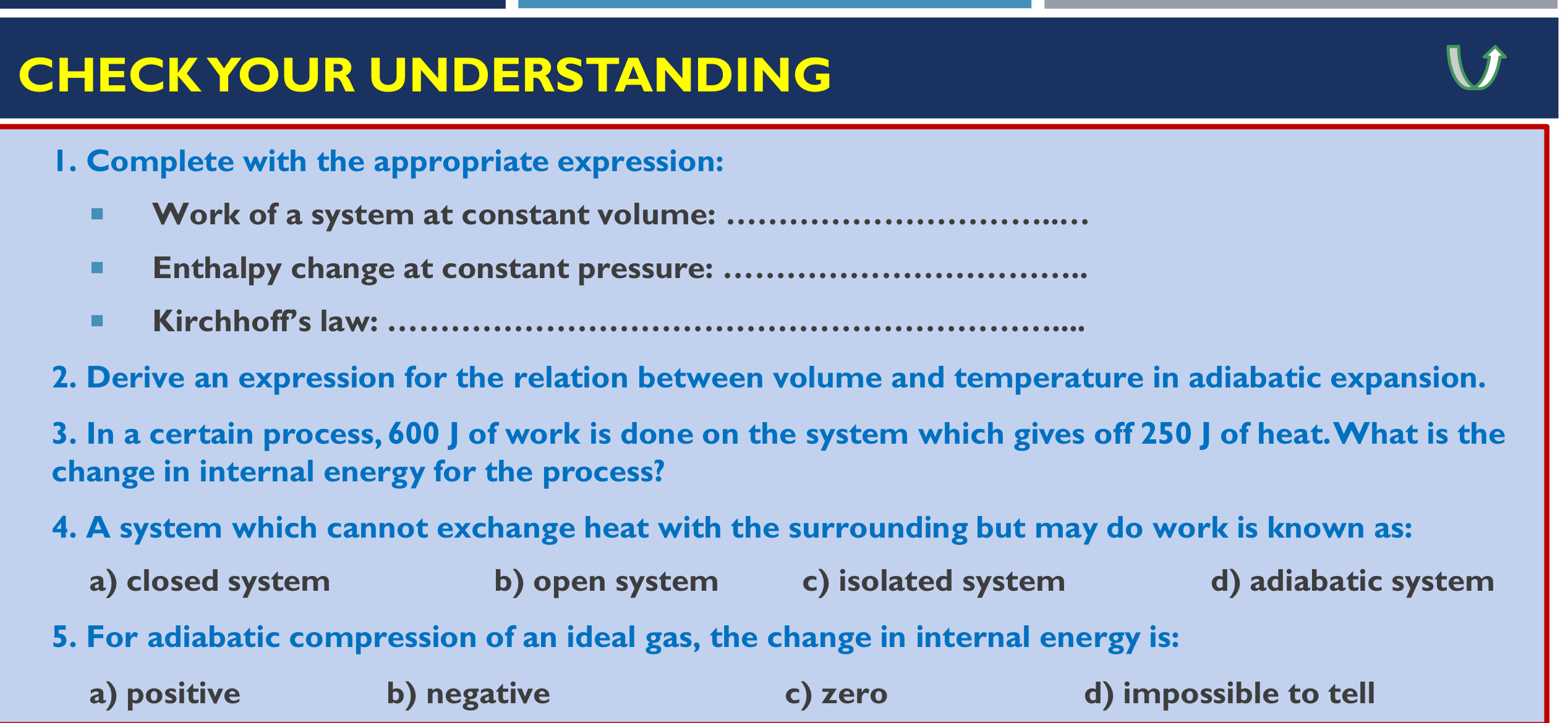
Question: Derive an expression for the relation between volume and temperature in adiabatic expansion. In a certain process, 6 0 0 J of work is done
Derive an expression for the relation between volume and temperature in adiabatic expansion.
In a certain process, of work is done on the system which gives off of heat. What is the
change in internal energy for the process?
A system which cannot exchange heat with the surrounding but may do work is known as:
a closed system
b open system
c isolated system
d adiabatic system
For adiabatic compression of an ideal gas, the change in internal energy is:
a positive
b negative
c zero
d impossible to tell
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


