Question: + Deriving Gas Law Formulas Learning Goal: To understand how to determine the appropriate formula for a given gas law problem. When you are
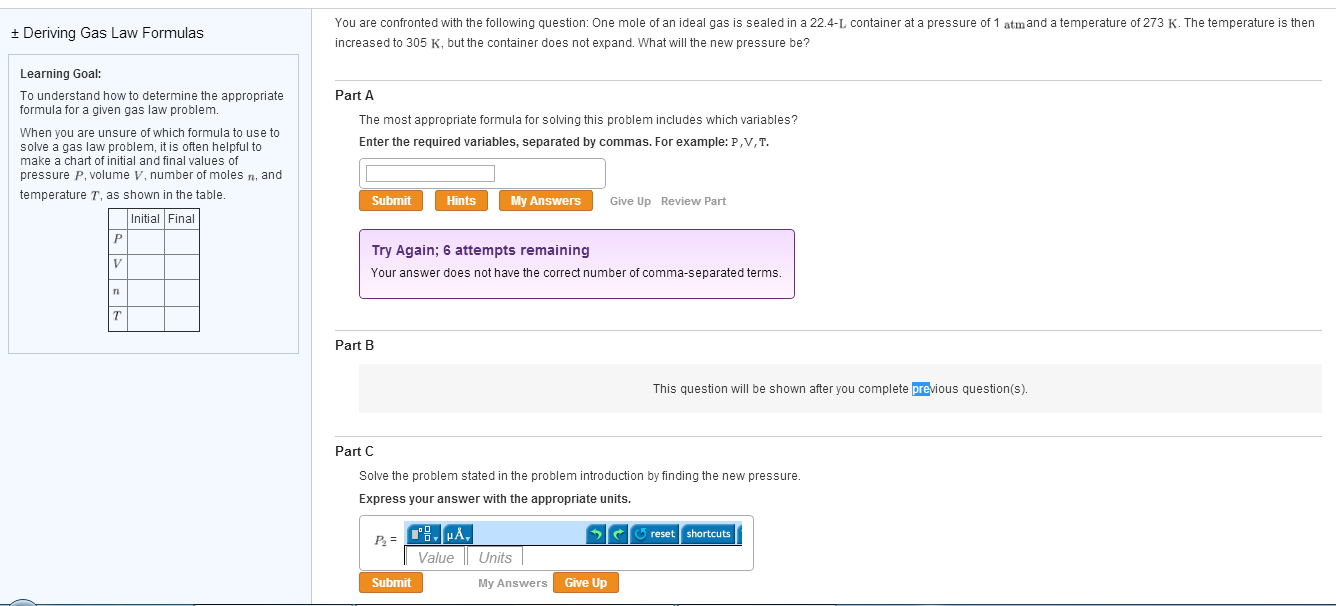
+ Deriving Gas Law Formulas Learning Goal: To understand how to determine the appropriate formula for a given gas law problem. When you are unsure of which formula to use to solve a gas law problem, it is often helpful to make a chart of initial and final values of pressure P, volume V, number of moles , and temperature T, as shown in the table. Initial Final P V 71 n T You are confronted with the following question: One mole of an ideal gas is sealed in a 22.4-L, container at a pressure of 1 atmand a temperature of 273 K. The temperature is then increased to 305 K, but the container does not expand. What will the new pressure be? Part A The most appropriate formula for solving this problem includes which variables? Enter the required variables, separated by commas. For example: P,V, T. Submit Part B Hints Try Again; 6 attempts remaining Your answer does not have the correct number of comma-separated terms. My Answers P = 8, A Submit Part C Solve the problem stated in the problem introduction by finding the new pressure. Express your answer with the appropriate units. Give Up Review Part Value Units My Answers Give Up This question will be shown after you complete previous question(s). reset shortcuts
Step by Step Solution
3.54 Rating (164 Votes )
There are 3 Steps involved in it
P Solution P 15m P V 224 L 224 cm V 224L Volume does n... View full answer

Get step-by-step solutions from verified subject matter experts


