Question: 39. A student titrated a 10.0 mL sample of nitric acid with a 1.50 mol/L sodium hydroxide solution in the presence of an indicator.
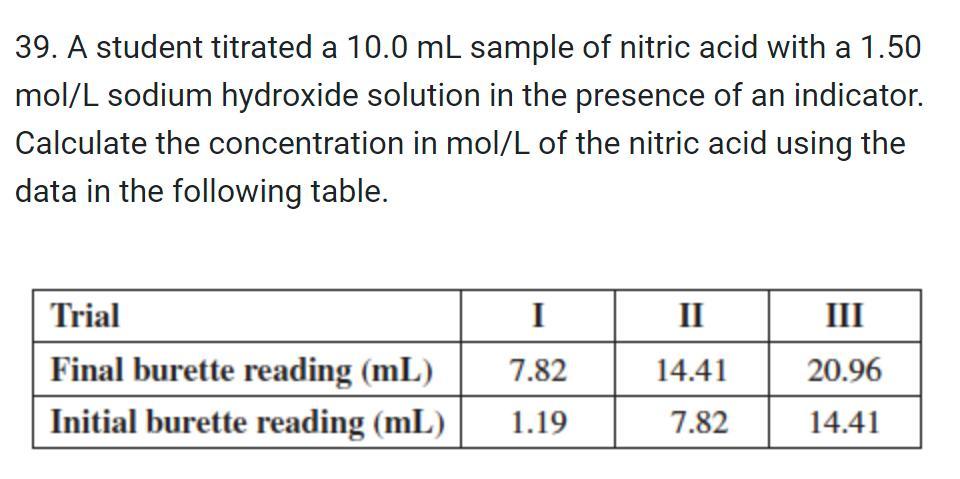
39. A student titrated a 10.0 mL sample of nitric acid with a 1.50 mol/L sodium hydroxide solution in the presence of an indicator. Calculate the concentration in mol/L of the nitric acid using the data in the following table. Trial Final burette reading (mL) Initial burette reading (mL) I 7.82 1.19 II 14.41 7.82 III 20.96 14.41
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
Answer The concentration in molL of the nitric aci... View full answer

Get step-by-step solutions from verified subject matter experts


