Question: Describe in practical terms how you would prepare 100 ml of 0.5 M Tris-HCl buffer (pH = 8.0) containing 0.1% SDS from the reagents listed
Describe in practical terms how you would prepare 100 ml of 0.5 M Tris-HCl buffer (pH = 8.0) containing 0.1% SDS from the reagents listed on p. 8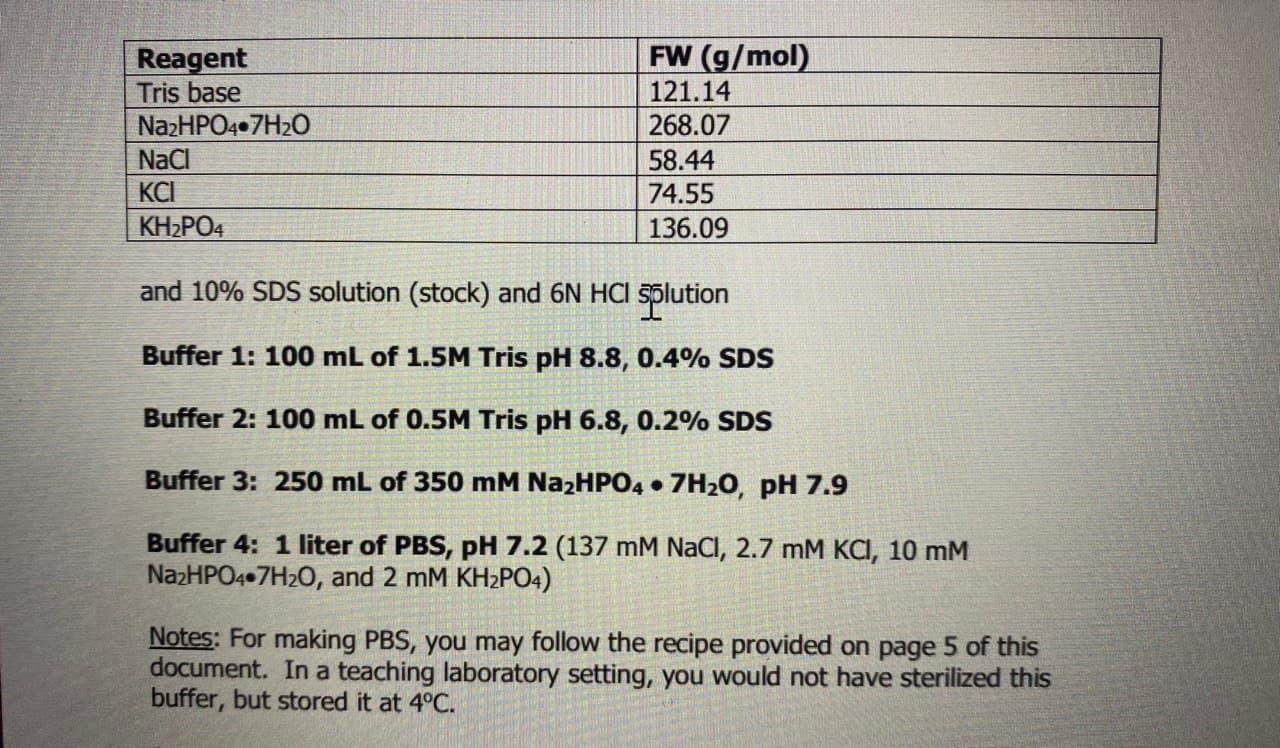
Reagent Tris base Na2HPO4.7H2O Naci KH2PO4 FW (g/mol) 121.14 268.07 58.44 74.55 136.09 and 10% SDS solution (stock) and 6N HCI solution Buffer 1: 100 mL of 1.5M Tris pH 8.8, 0.4% SDS Buffer 2: 100 mL of 0.5M Tris pH 6.8, 0.2% SDS Buffer 3: 250 mL of 350 mM Na2HPO4.7H20, pH 7.9 Buffer 4: 1 liter of PBS, pH 7.2 (137 mM NaCl, 2.7 mM KCI, 10 MM NazHPO4.7H2O, and 2 mM KH2PO4) Notes: For making PBS, you may follow the recipe provided on page 5 of this document. In a teaching laboratory setting, you would not have sterilized this buffer, but stored it at 4C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


