Question: Determine an unknown volume or concentration using an oxidation-reduction titration. The concentration of a SO32 solution is determined by titrating it with a 0.1921M solution
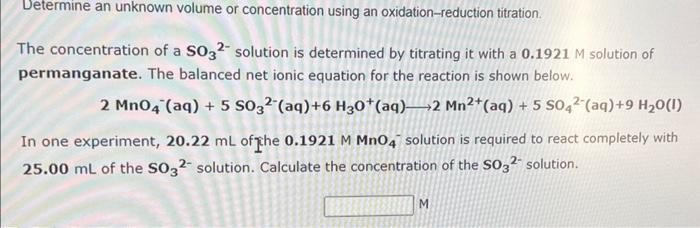
Determine an unknown volume or concentration using an oxidation-reduction titration. The concentration of a SO32 solution is determined by titrating it with a 0.1921M solution of permanganate. The balanced net ionic equation for the reaction is shown below. 2MnO4(aq)+5SO32(aq)+6H3O+(aq)2Mn2+(aq)+5SO42(aq)+9H2O(l) In one experiment, 20.22mL of che 0.1921MMnO4solution is required to react completely with 25.00mL of the SO32 solution. Calculate the concentration of the SO32 solution. M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


