Question: Determine if most reactants or products will exist at equilibrium based on the magnitude of the equilibrium constant. a. N 2(g) + O 2(g) 2
Determine if most reactants or products will exist at equilibrium based on the magnitude of the equilibrium constant.
a. N2(g) + O2(g) 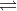 2 NO(g) Kc = 1.5 X 10-10
2 NO(g) Kc = 1.5 X 10-10
b. 2 SO2(g) + O2(g) 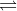 2 SO3(g) Kc = 6.1 1010
2 SO3(g) Kc = 6.1 1010
c. 2 NO(g) + O2(g) 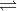 2 NO2(g) Kc = 1.2 1014
2 NO2(g) Kc = 1.2 1014
d. 2 HBr(g) 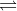 H2(g) + Br2(g) Kc = 5.8 10-18
H2(g) + Br2(g) Kc = 5.8 10-18
d. 2 HBr(g) H2(g) + Br2(g) Kc = 5.8 10-18
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


