Question: Determine the electron geometry for each molecule. Drag the items into the appropriate bins. Linear CH,Br SBr BCls PF Trigonal planar Tetrahedral Reset Help
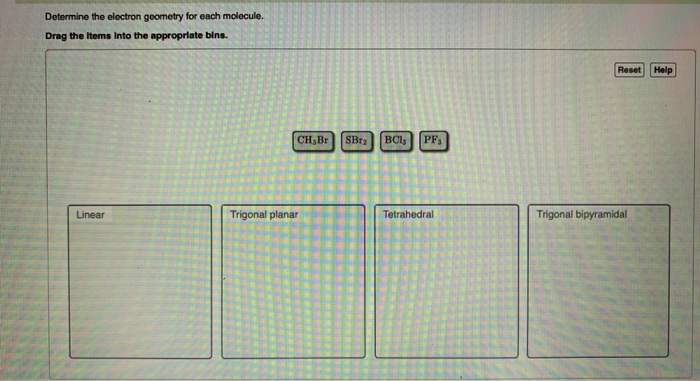
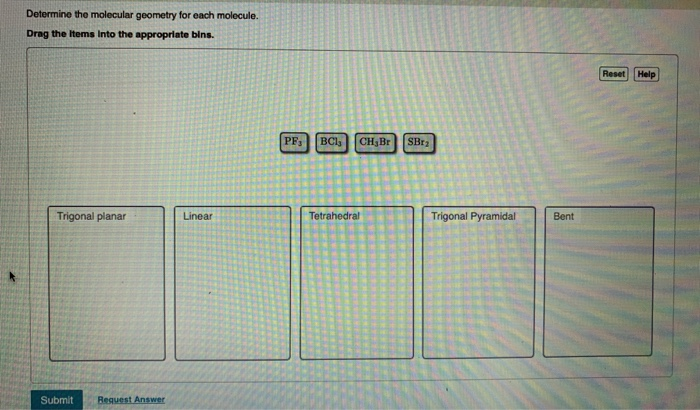
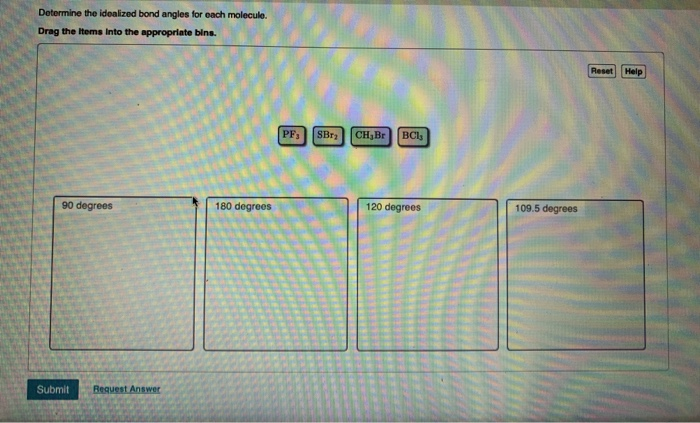
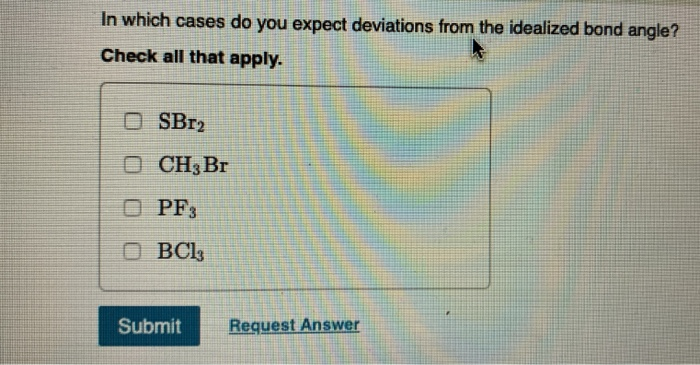
Determine the electron geometry for each molecule. Drag the items into the appropriate bins. Linear CH,Br SBr BCls PF Trigonal planar Tetrahedral Reset Help Trigonal bipyramidal Determine the molecular geometry for each molecule. Drag the items Into the appropriate bins. Trigonal planar Submit Request Answer Linear PF BC1 CH Br Tetrahedral SBr Trigonal Pyramidal Bent Reset Help Determine the idealized bond angles for each molecule. Drag the Items Into the appropriate bins. 90 degrees Submit Request Answer 180 degrees PF3 SBr CH Br BC13 120 degrees 109.5 degrees Reset Help In which cases do you expect deviations from the idealized bond angle? Check all that apply. OSBr2 OCH3 Br OPF3 OBC13 Submit Request Answer
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


