Question: Determine the energy that would be generated by the amount of analyte remaining after 1.5 h at 23C, knowing that K = 10.21 and S
Determine the energy that would be generated by the amount of analyte remaining after 1.5 h at 23C, knowing that K = 10.21 and S = -3202.41 J K-1 mol-1 molecular weight of analyte = 234.05 g mol-1
moles = 9.5x10^(-4) mmol
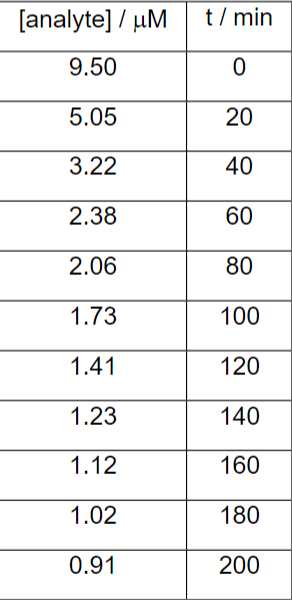
2nd order kinetics - rate constant = 4.93x10^(-3) u / m.min
[analyte] / uM t/min 9.50 0 5.05 20 3.22 40 2.38 60 2.06 80 1.73 100 1.41 120 1.23 140 1.12 160 1.02 180 0.91 200
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


