Question: Determine the van't Hoff factor (i) for the following solutions. Assume 100% dissociation for ionic solutes. 0.26MNaF;i=0.0040MSrBr2;i= 1.100MC2H6O2;i= 0.061MCa(NO3)2;i=
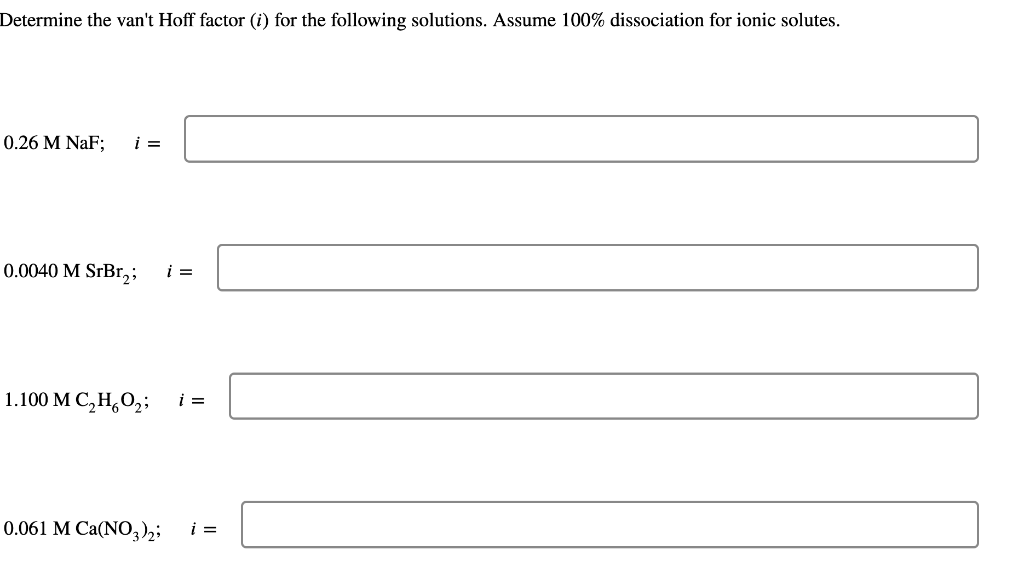
Determine the van't Hoff factor (i) for the following solutions. Assume 100% dissociation for ionic solutes. 0.26MNaF;i=0.0040MSrBr2;i= 1.100MC2H6O2;i= 0.061MCa(NO3)2;i=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


