Question: Determine whether the products or reactants are higher in entropy, then predict the sign of Srxn. reactants are higher in entropy; S is negative reactants
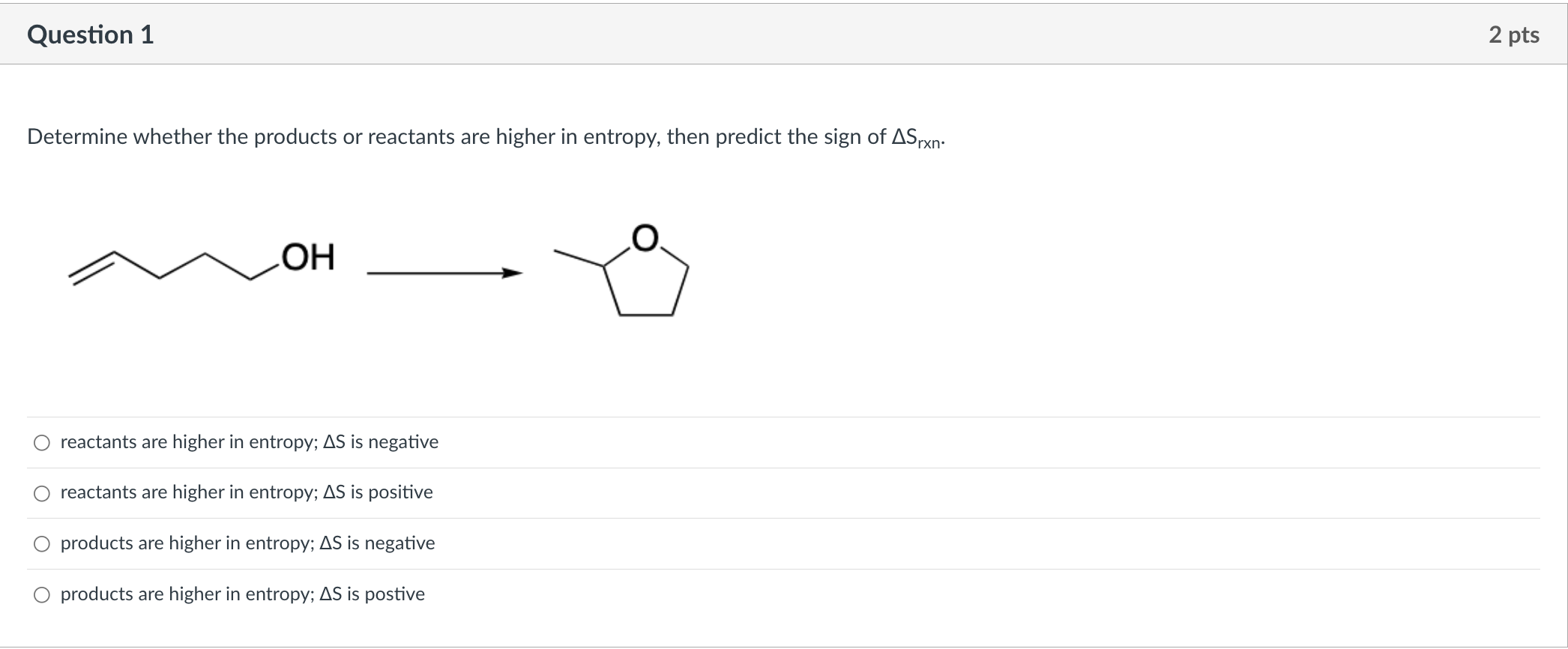
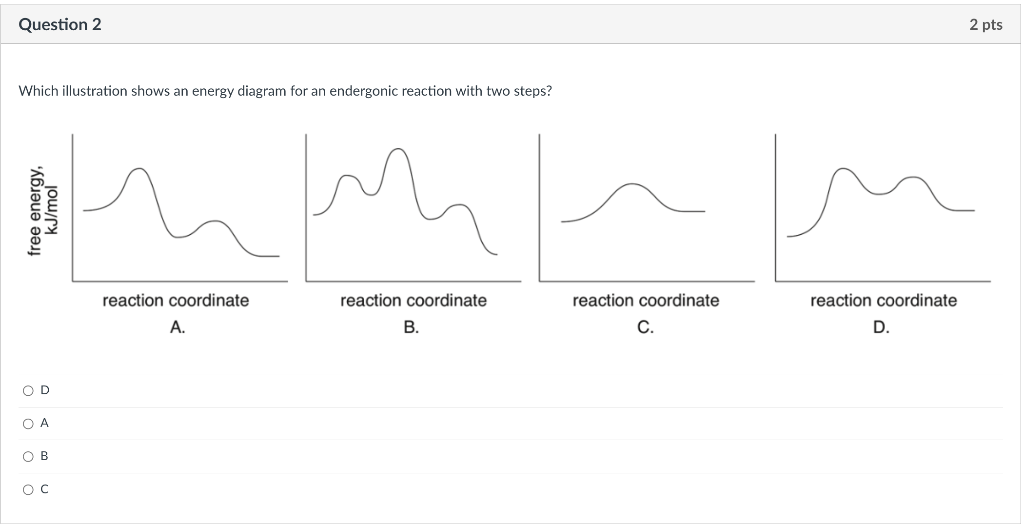
Determine whether the products or reactants are higher in entropy, then predict the sign of Srxn. reactants are higher in entropy; S is negative reactants are higher in entropy; S is positive products are higher in entropy; S is negative products are higher in entropy; S is postive Which illustration shows an energy diagram for an endergonic reaction with two steps
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


