Question: Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the
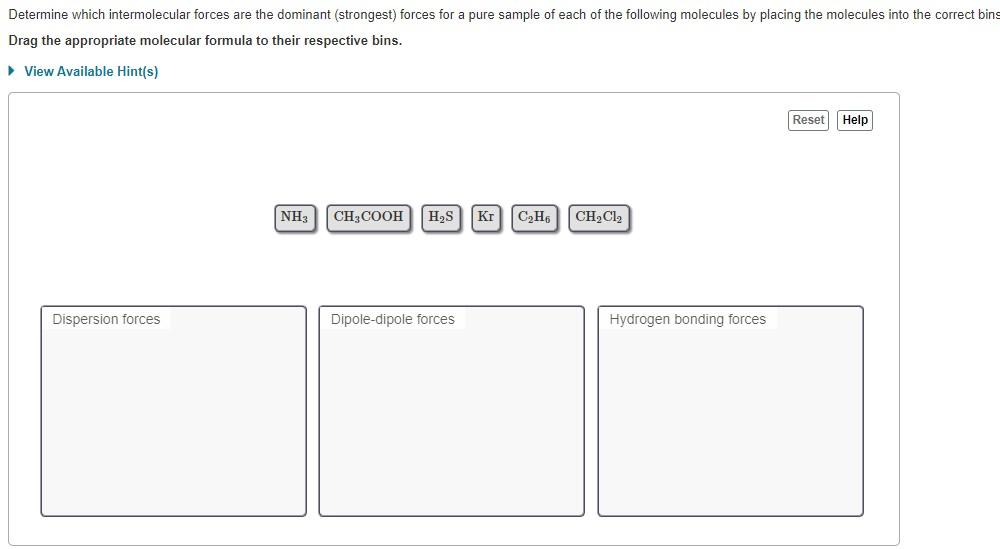
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins Drag the appropriate molecular formula to their respective bins. View Available Hint(s) Reset Help NH3 CH3COOH HZS Kr C2H61 CH2Cl2 Dispersion forces Dipole-dipole forces Hydrogen bonding forces
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


