Question: Determining rate law from time and concentration data. (Use the integrated rate laws and graphing to get orders). 4a. The rate of this rxn depends
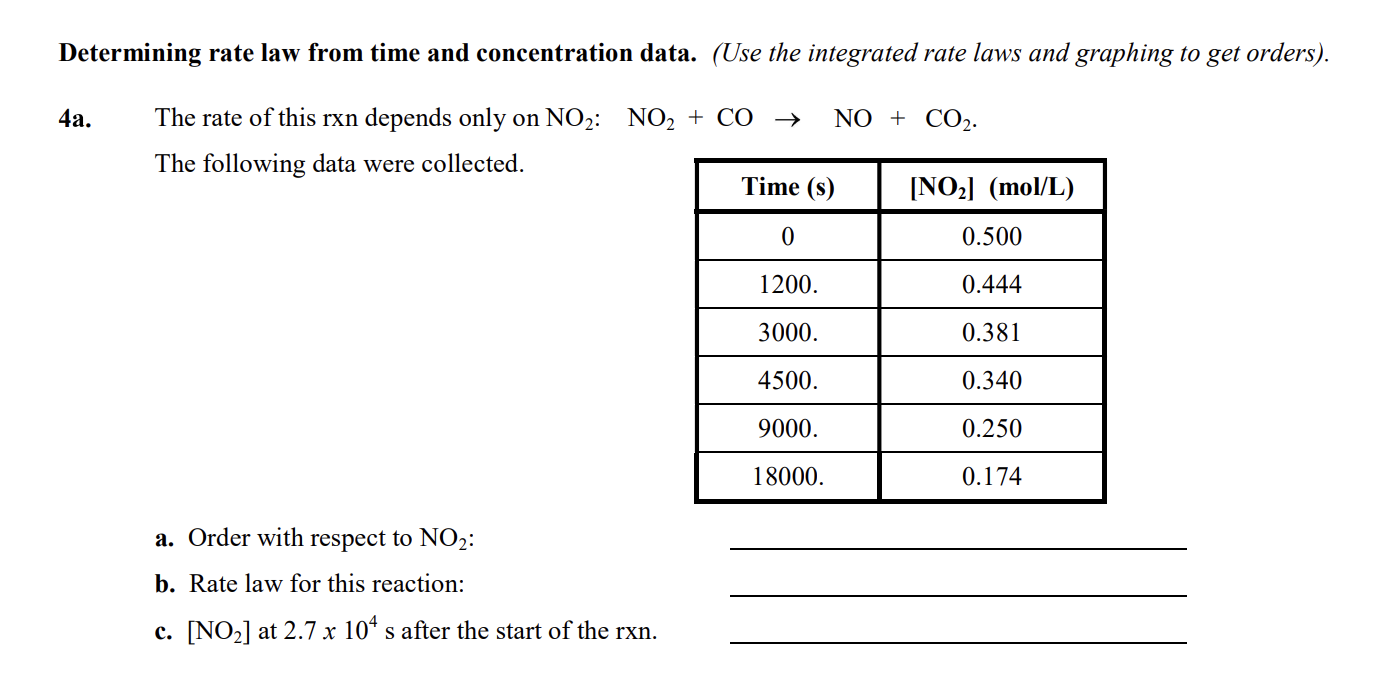
Determining rate law from time and concentration data. (Use the integrated rate laws and graphing to get orders). 4a. The rate of this rxn depends only on NO2:NO2+CONO+CO2. The following data were collected. a. Order with respect to NO2 : b. Rate law for this reaction: c. [NO2] at 2.7104s after the start of the rxn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


