Question: Develop a Porter's Five Forces Model for the following Company wthe regarding informatio. . THREAT OF NEW ENTRANTS . 1 RIVALRY AMONG EXISTING COMPETITORS: Number
 Develop a Porter's Five Forces Model for the following Company wthe regarding informatio.
Develop a Porter's Five Forces Model for the following Company wthe regarding informatio.

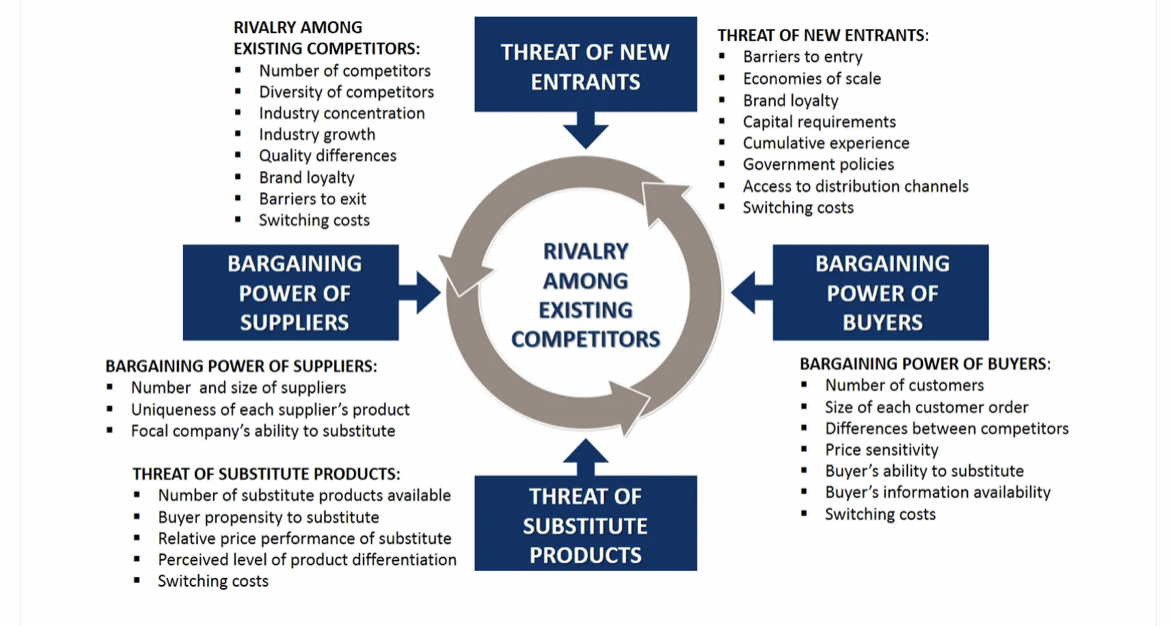
. THREAT OF NEW ENTRANTS . 1 RIVALRY AMONG EXISTING COMPETITORS: Number of competitors Diversity of competitors . Industry concentration Industry growth Quality differences Brand loyalty . Barriers to exit Switching costs THREAT OF NEW ENTRANTS: Barriers to entry Economies of scale Brand loyalty . Capital requirements Cumulative experience Government policies Access to distribution channels Switching costs 1 . . BARGAINING POWER OF SUPPLIERS RIVALRY AMONG EXISTING COMPETITORS BARGAINING POWER OF BUYERS BARGAINING POWER OF SUPPLIERS: Number and size of suppliers Uniqueness of each supplier's product Focal company's ability to substitute . BARGAINING POWER OF BUYERS: Number of customers Size of each customer order Differences between competitors Price sensitivity Buyer's ability to substitute Buyer's information availability Switching costs . THREAT OF SUBSTITUTE PRODUCTS: Number of substitute products available Buyer propensity to substitute Relative price performance of substitute Perceived level of product differentiation Switching costs THREAT OF SUBSTITUTE PRODUCTS . Clinigene Emboldened by the strength of Biocon India's culture and its two subsidiaries, Mazumdar-Shaw and her senior team developed a vision: to become a fully integrated drug discovery and development company. The Biocon India Group already possessed or was developing the capabilities for conducting research and development, manufacturing pharmaceuticals, and marketing its products. Besides animal testing, Biocon's missing link in the traditional pharmaceutical value chain was the ability to run clinical trials (see Exhibit 2).12 Thus in the year 2000 Biocon India launched a new subsidiary: Clinigene. Clinigene sought ultimately to offer a broad range of clinical trial services, recognizing that drug development could span two different areas that consequently required different types of clinical studies. Generally, generic drugs required bio-equivalence and bio-availability (BE/BA) clinical studies to prove that the generic drug worked as well as the off-patent original drug. But for new drugs, much more elaborate clinical trials had to be conducted. In the few years since its launch, Clinigene had focused not on organizing trials but on clinical lab services, BE/BA studies, and partnership coordination with hospitals. As Chief Operating Officer Dr. A. S. Arvind noted, By building up capabilities in conducting BE/BA studies and clinical trials, Clinigene fills a key missing gap in the drug discovery and development value chain for Biocon. According to Dr. Nadig, Vice President of Medical Services, the services contributed to Clinigene's ability to conduct high quality clinical research from start to finish. Yet launching Clinigene raised multiple concerns, largely because it was not clear how soon Biocon India Group would need its capabilities. Biocon India was still several years away from developing its own drug molecules. Rather than put Clinigene on hold until in-house demand kicked in, Mazumdar-Shaw expected Clinigene to sustain itself with external clients in the CRO business. More than two years after Clinigene's creation, doubts remained about the risks it posed, risks particularly in market positioning, culture, publicity, and ethics. This high growth potential could represent significant opportunity for Clinigene to reap revenues as a CRO player. On the other hand, Clinigene would have to position itself carefully. Indian CROs were focused primarily on serving the need for BE/BA studies in the market, and although a few were beginning to offer services in clinical trials, some pharmaceutical MNCs were wary of outsourcing such critical and sensitive tasks to a largely unproven Indian industry. Meanwhile foreign CROS, such as Quintiles, and the in-house data management centers of big pharmaceutical companies, such as GlaxoSmithKline and Pfizer, were focusing their efforts on serving higher-value needs of the market, particularly data management and Phase III clinical trials (see Exhibit 3). Clinigene's current capabilities positioned it in the low- to medium-value segment of the value chain. Moving up the value chain might be more profitable in the long run but would entail significant costs, both financial and cultural. Organizational Culture Mazumdar-Shaw and her team also recognized that Biocon India Group could end up a victim of Clinigene's success. Were it to succeed in capturing a significant slice of a growing market, Clinigene could conceivably grow to a size that overshadowed Biocon and Syngene. Such aggressive growth meant the diversion of time and resources. It also meant a large influx of new employees and little time to inculcate the Biocon culture particularly the two years cited by Shri Suryanarayan. New organizational designs and procedures might need to be introduced and enforced, in a company that prided itself on loose structures and casual hierarchies. Moreover, the nature of clinical trials required that much of the work be done in partner hospitals. Physically dispersed among trial sites, rarely able to wander the corridors or enjoy Biocons facilities, the Clinigene employees could easily end up disaffected, or at least loyal only to Clinigene rather than its parent Group. Publicity and Ethics Clinical trials dealt with humans, and thus carried significant risk to the CRO sponsoring the clinical trial. Although rigorous and stringent conditions were imposed by the industry and government bodies, the risk still fell upon the company running the experiments. Furthermore, this risk could
 Develop a Porter's Five Forces Model for the following Company wthe regarding informatio.
Develop a Porter's Five Forces Model for the following Company wthe regarding informatio.





