Question: Device 1, shown at right, represents a heat engine operating between reservoirs at 900 K and 400 K. (The system interacting with the reservoirs
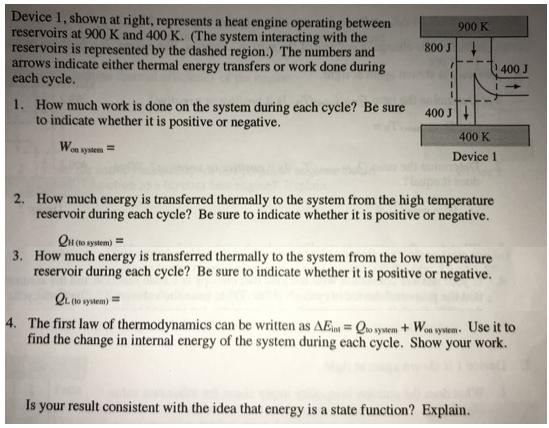
Device 1, shown at right, represents a heat engine operating between reservoirs at 900 K and 400 K. (The system interacting with the reservoirs is represented by the dashed region.) The numbers and arrows indicate either thermal energy transfers or work done during each cycle. 900 K 800 J 400 J 1. How much work is done on the system during each cycle? Be sure to indicate whether it is positive or negative. 400 J 400 K Wa wysem= Device 1 2. How much energy is transferred thermally to the system from the high temperature reservoir during each cycle? Be sure to indicate whether it is positive or negative. Qt to system) = 3. How much energy is transferred thermally to the system from the low temperature reservoir during each cycle? Be sure to indicate whether it is positive or negative. Qu to ytem) = 4. The first law of thermodynamics can be written as AE = Qo sysem + Won wystem. Use it to find the change in internal energy of the system during each cycle. Show your work. Is your result consistent with the idea that energy is a state function? Explain.
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
To solve these problems lets refer to the given diagram and apply the principles of thermodynamics 1 ... View full answer

Get step-by-step solutions from verified subject matter experts


