Question: Directions: Solve the following problem using the GRADS-IDEA method neatly and legibly. Then, scan and upload your handwritten file or type your response and upload
Directions: Solve the following problem using the GRADS-IDEA method neatly and legibly. Then, scan and upload your handwritten file or type your response and upload as a word document. Saccharomyces cerevisiae is a species of yeast that has been used extensively in baking and brewing since antiquity and is now the "model eukaryote" in the same way that E. coli is the "model bacterium". Much has been learned and characterized about its metabolism and byproducts. Environmental conditions affect its metabolism in a similar manner to other eukaryotes; it can survive and grow in plentiful oxygen or deficient oxygen conditions. These are known as aerobic and anaerobic metabolism, respectively. Bakers, brewers, and vintners (winemakers) prefer anaerobic respiration, because anaerobic respiration consumes glucose to produce ethanol and carbon dioxide (among other products) while aerobic respiration consumes ethanol to produce biomass (among other products). In baking, carbon dioxide causes the bread to rise and whatever ethanol is generated boils off while both ethanol and carbon dioxide remain in brews to increase ABV and carbonation, respectively. a) Use MATLAB to balance the following metabolic equations for S. cerevisiae by solving for the appropriate unknowns. (Attach a screenshot of your workspace showing your work. Although everything can be done in the workspace, if you do write a script or function to assist you, be sure to attach the code as a .txt or .m file.) Aerobic - aC2H5OH + bNH3 + cO2 pCH1.704N0.149O0.408 + qCO2 + rH2O, RQ = 0.66 [Ethanol + Ammonia + Oxygen Biomass + Carbon Dioxide + Water] Anaerobic - aC6H12O6 + bNH3 0.59CH1.74N0.2O0.45 + 1.3C2H5OH + qC3H8O3 + rCO2 + sH2O [Glucose + Ammonia Biomass + Ethanol + Ethylene Glycol + Carbon Dioxide + Water] You are operating an immobilized yeast reactor in which yeast are sandwiched between two finely-porous plates of silicon carbide ceramic that allow reactants, products, and solvents to pass through while yeast are kept stationary between the ceramic plates. The reaction vessel has two modes of operation: continuous and looped. Continuous operation passes reactants through the yeast to achieve an arbitrary ABV level. There is an inlet for an aqueous glucose stream, an aqueous ammonia stream, and an outlet for any products and remaining reactants. In looped mode, fermentation beyond the initial ABV of ethanol is achieved by reprocessing the outlet stream until the desired ethanol concentration is reached. As mentioned above, brewers and vintners prefer anaerobic respiration, so oxygen is scavenged out of the inlet stream(s) so that the concentration of oxygen in the reactor can be considered negligible under ideal conditions. b) Using the balanced equation for anaerobic respiration in part (a) and the information below, determine: i. the limiting reactant and fractional conversion of the other reactant ii. the mass generated of biomass and the composition of the outlet stream iii. the final ABV if the glucose and ammonia are carried into the reactor by 16 L/hr of water
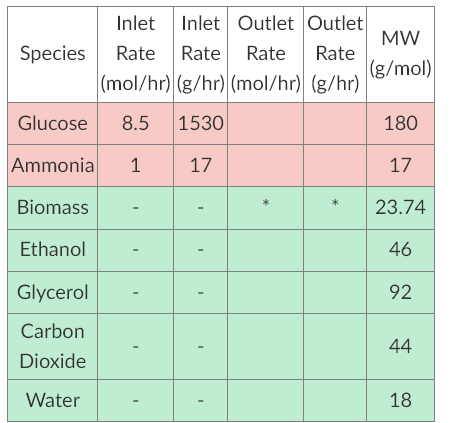
*Biomass does not leave, but is generated during the reaction. HINT: You will need to find chemical property values that are not given. List them in your assumptions.
\begin{tabular}{|c|c|c|c|c|c|} \hline Species & InletRate(mol/hr) & InletRate(g/hr) & OutletRate(mol/hr) & OutletRate(g/hr) & MW(g/mol) \\ \hline Glucose & 8.5 & 1530 & & & 180 \\ \hline Ammonia & 1 & 17 & & & 17 \\ \hline Biomass & - & - & & & 23.74 \\ \hline Ethanol & - & - & & & 46 \\ \hline Glycerol & - & - & & & 92 \\ \hline CarbonDioxide & - & - & & & 44 \\ \hline Water & - & - & & & 18 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


