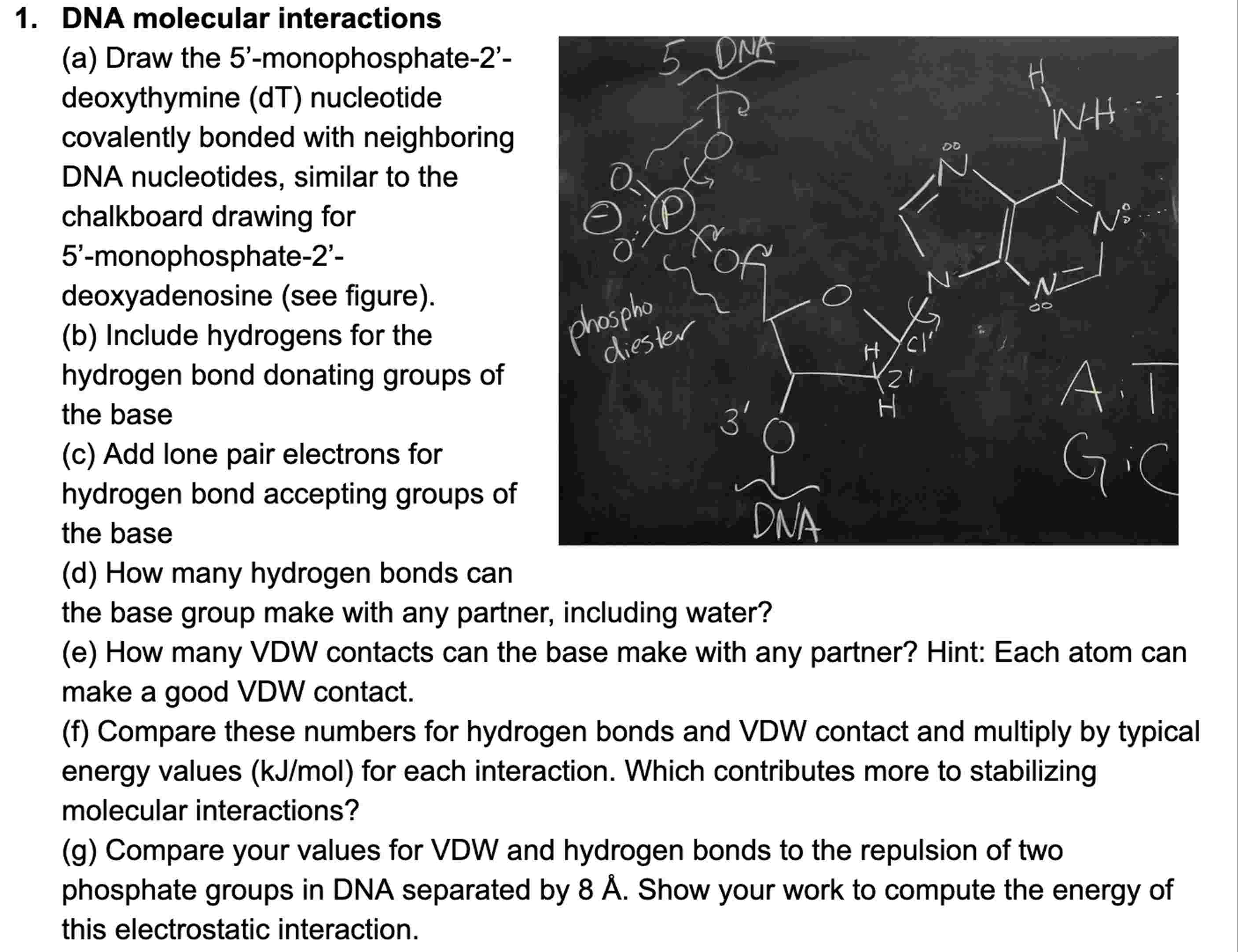
Question: DNA molecular interactions ( a ) Draw the 5 ' - monophosphate - 2 ' - deoxythymine ( dT ) nucleotide covalently bonded with neighboring
DNA molecular interactions
a Draw the monophosphate
deoxythymine dT nucleotide
covalently bonded with neighboring
DNA nucleotides, similar to the
chalkboard drawing for
monophosphate
deoxyadenosine see figure
b Include hydrogens for the
hydrogen bond donating groups of
the base
c Add lone pair electrons for
hydrogen bond accepting groups of
the base
d How many hydrogen bonds can
the base group make with any partner, including water?
e How many VDW contacts can the base make with any partner? Hint: Each atom can
make a good VDW contact.
f Compare these numbers for hydrogen bonds and VDW contact and multiply by typical
energy values for each interaction. Which contributes more to stabilizing
molecular interactions?
g Compare your values for VDW and hydrogen bonds to the repulsion of two
phosphate groups in DNA separated by Show your work to compute the energy of
this electrostatic interaction.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


