Question: do it according to the question . pls don't take others work and do it by yourself. if not i will be report to chegg.
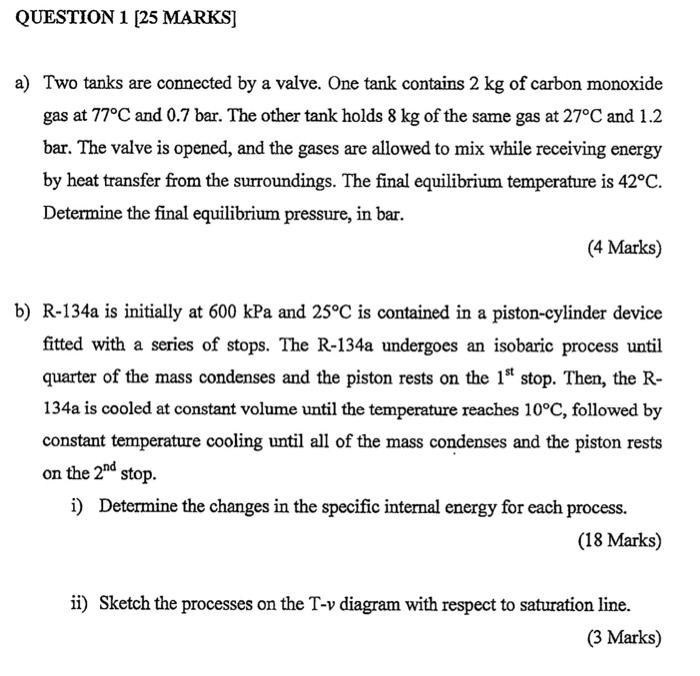
QUESTION 1 (25 MARKS] a) Two tanks are connected by a valve. One tank contains 2 kg of carbon monoxide gas at 77C and 0.7 bar. The other tank holds 8 kg of the same gas at 27C and 1.2 bar. The valve is opened, and the gases are allowed to mix while receiving energy by heat transfer from the surroundings. The final equilibrium temperature is 42C. Determine the final equilibrium pressure, in bar. (4 Marks) b) R-134a is initially at 600 kPa and 25C is contained in a piston-cylinder device fitted with a series of stops. The R-134a undergoes an isobaric process until quarter of the mass condenses and the piston rests on the 1st stop. Then, the R- 134a is cooled at constant volume until the temperature reaches 10C, followed by constant temperature cooling until all of the mass condenses and the piston rests on the 2nd stop. i) Determine the changes in the specific internal energy for each process. (18 Marks) ii) Sketch the processes on the T-v diagram with respect to saturation line
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


