Question: DO NOT answer part B, only answer this part of the question PART A , as part B is not posted here. I only want
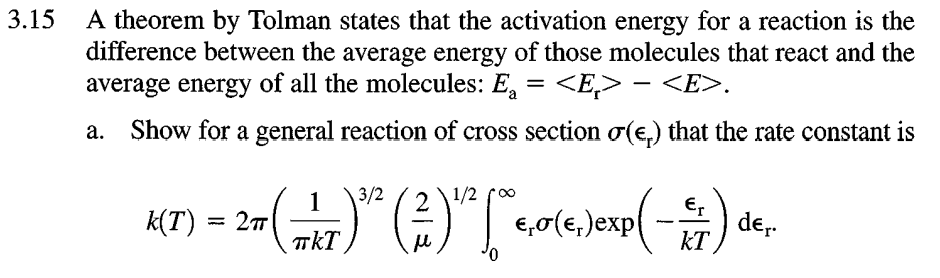 DO NOT answer part B, only answer this part of the question PART A, as part B is not posted here. I only want part A, and I want all of the steps written out. If you can't do that DO NOT ANSWER thank you :)
DO NOT answer part B, only answer this part of the question PART A, as part B is not posted here. I only want part A, and I want all of the steps written out. If you can't do that DO NOT ANSWER thank you :)
3.15 A theorem by Tolman states that the activation energy for a reaction is the difference between the average energy of those molecules that react and the average energy of all the molecules: E.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


