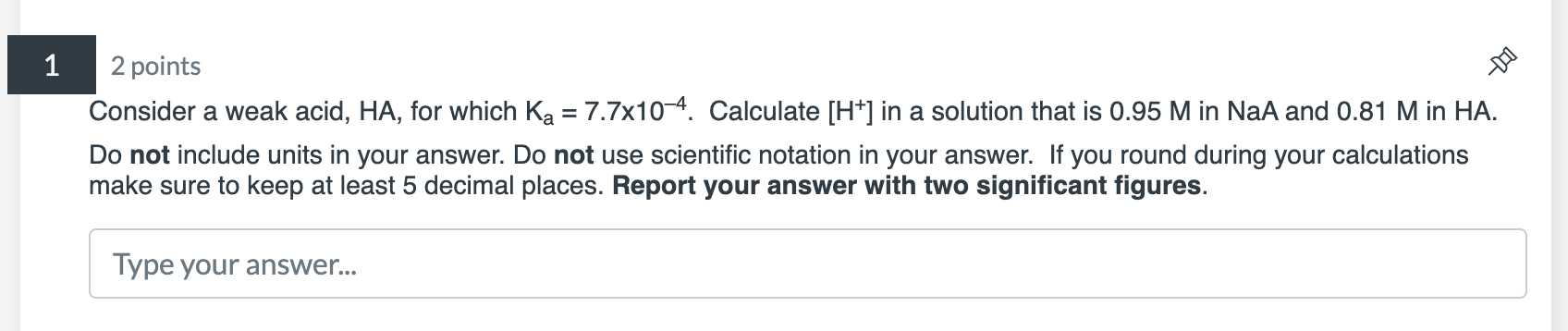
Question: do not show work, answer only please Consider a weak acid, HA, for which Ka=7.7104. Calculate [H+]in a solution that is 0.95M in NaA and
do not show work, answer only please
Consider a weak acid, HA, for which Ka=7.7104. Calculate [H+]in a solution that is 0.95M in NaA and 0.81M in HA. Do not include units in your answer. Do not use scientific notation in your answer. If you round during your calculations make sure to keep at least 5 decimal places. Report your answer with two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


