Question: DO NOT SOLVE 1A: ANSWER IS 0.0003, USE THIS TO SOLVE 1B PLEASE!!! 1A) Consider the reaction A ---> B with G = 20 KJ/mol.
DO NOT SOLVE 1A: ANSWER IS 0.0003, USE THIS TO SOLVE 1B PLEASE!!!
1A) Consider the reaction A ---> B with G = 20 KJ/mol. What is the equilibrum ratio of [B]/[A] at 25C? 0.0003 is answer
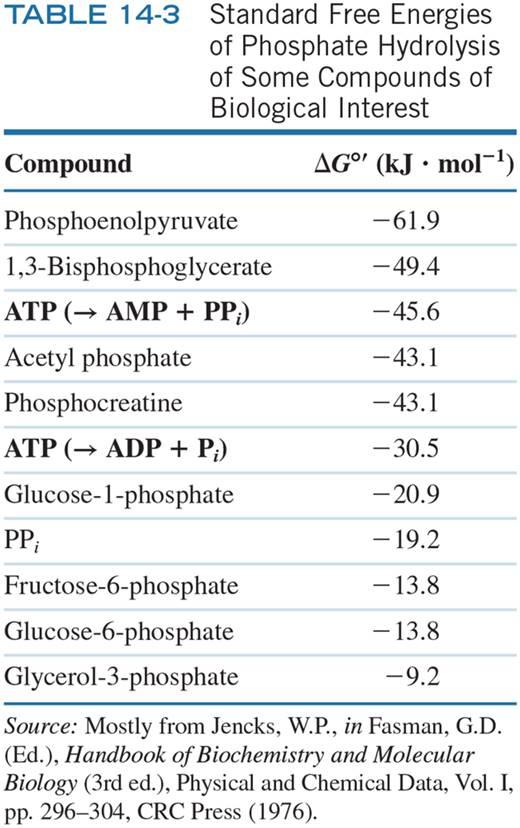
1B) Using the information from Question 1A ... Suppose that you couple this reaction with the hydrolysis of ATP to ADP and Pi. What is the equilibrium ratio of [B]/[A] at 25C if the concentrations all the other species (ATP, ADP, & Pi) are 1M?
(Ed.), Handbook of Blochemistry and Molecular Biology (3rd ed.), Physical and Chemical Data, Vol. I, pp. 296-304, CRC Press (1976)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


