Question: Do some additional online research on Direct to Consumer Pharma Advertising and Snow Companies related to the questions posed at the end of the case.
Do some additional online research on "Direct to Consumer Pharma Advertising" and "Snow Companies" related to the questions posed at the end of the case. Do an in-depth analysis and answer the 5 questions at the end of the case. Each question to be answered separately, References and links at the end of your answers.
Very few marketers have more ethical and legal regulations imposed on their marketing activities than those who operate in the pharmaceutical and biotech industry. This industry is highly regulated, both externally and internally. Compliance is the name of the game.
Just think of the sophisticated web of key players in this industry and how each player affects the actions of another. Those key players include patients, doctors, pharmaceutical companies, medical trade associations, insurance companies, and the government.
The need for ethical use of patients medical information is quite obvious in this industry, as each patients medical records are private. These are highly sensitive documents that must be kept strictly confidential. However, the barrage of legal restrictions imposed on the marketing of branded pharmaceutical drugs is simply mind-boggling. This case will present the challenges and marketing strategies associated with pharmaceutical marketing.
The ethical and legal regulations associated with pharmaceutical marketing make business as usual vastly different from that of nearly any other industry.
With a decrease in the variety of marketing activities legally and ethically permitted by pharmaceutical sales reps visiting doctors, many pharmaceutical marketers are now placing more emphasis on direct-to- consumer (DTC) marketing. Investments in direct marketing activities in this industry have been rising for decades and are expected to continue to grow in the future.
Direct-To-Consumer (DTC) Marketing
Have you ever wondered about the drug your doctor prescribed to you or a loved one? Do you ever wish you could talk to someone who has a similar condition? Have you ever researched a disease or condition you or a loved one has in advance of a doctors visit to be better prepared to ask the right questions? If youve answered yes to any of these questions, you are among the hundreds of millions of people across the nation who have helped to make direct-to-consumer (DTC) or direct-to-patient (DTP) pharmaceutical and biotechnology marketing a burgeoning endeavor. DTC/DTP pharmaceutical advertising is considered to be any marketing communication for prescription drugs that directly targets the final consumer, or individual patient, as opposed to promotions that target the physicians who write prescriptions. DTC/DTP marketing is currently allowed only in the United States and New Zealand. Other countries allow a variety of unbranded educational programs, such as grants for support groups or disease-awareness events.
While most consumers desire more information on the products they take or have been prescribed, the U.S. Food and Drug Administration (FDA) closely regulates this type of communication. In addition, the industry body PhRMA has created specific guidelines which pharmaceutical companies must follow. PhRMAs Direct-to-Consumer Advertising about Prescription Medicines are guidelines specific to direct promotion to consumers of prescription medications. These guidelines address the use of actors in television and print advertisements, the content of advertisements, and the lead times before DTC advertising for a new product may begin.
Products in other industries that pose significant health and occupational risks to individuals do not have the same level of scrutiny as do pharmaceuticals. Before a promotional piece can be created for a pharmaceutical product, it needs to undergo an internal review by the pharmaceutical company. Typically, there is a review team for each brand or therapeutic area within the company. These review committees may be called different things:
Joint Review Committee (JRC)typically used when two companies co-promote a product Promotional Review Committee (PRC) Review Committee (RC)
Communication Committee Review (CCR).
Most review committees are made up of the same key playerslegal, medical, regulatoryeach tasked with different prime directives and each viewing the promotional material through the lens of their personal experience.
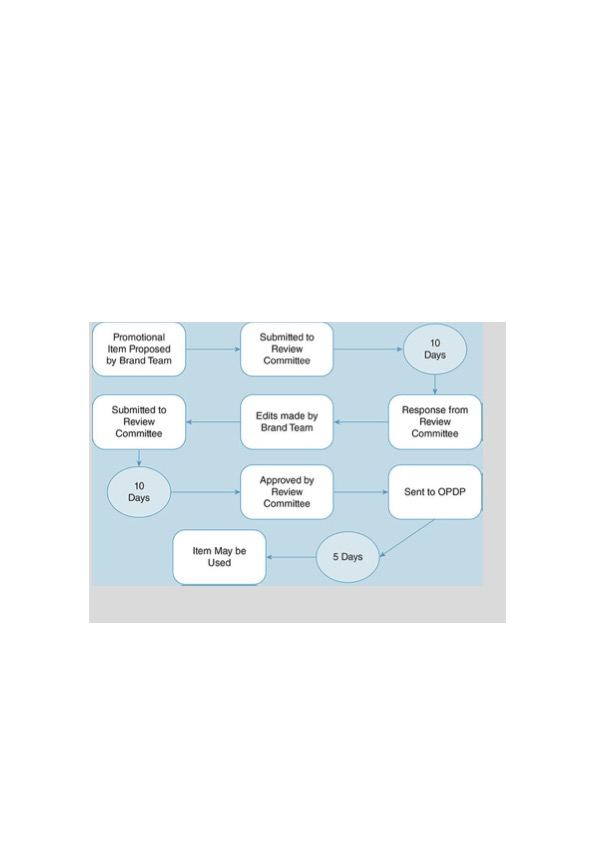
Each and every pharmaceutical advertisement or item that will be seen by either a physician or a consumer must go through an extensive and rigorous review process. Although the exact process varies by pharmaceutical company, the main point to be made is that getting a pharmaceutical advertisement through the review process entails a long and tedious process. An example of this detailed process is revealed inFigure 13.15. This process may take months and require several revisions before the ad or item obtains the necessary approval to be used in marketing.
Given the stringent federal regulations and scrutiny imposed on the direct marketing of pharmaceutical products, innovation is a prerequisite for success in this space. One company that has found a way to innovate and create value for clients is Snow Companies, a DTC/DTP and word-of- mouth healthcare marketing agency.
Figure 13.15 Review process flowchart. Used with permission of Snow & Associates.
Snow Companies Patient Ambassador Program
Brenda Snow, pictured in Figure 13.16, founded Snow Companies in 2001 and developed its proprietary Patient Ambassador program. After Snow was diagnosed with multiple sclerosis, she became frustrated at how little was being done to help educate, empower, and engage people like herself.
Snow Companies provides its clients with a wide variety of services, including graphic design and layout, account services, event management, video production and editing, marketing research, copy and creative writing, call center services, database management, and analytics. As can be seen in Figure 13.17, the companys services include live, digital, and print offerings.
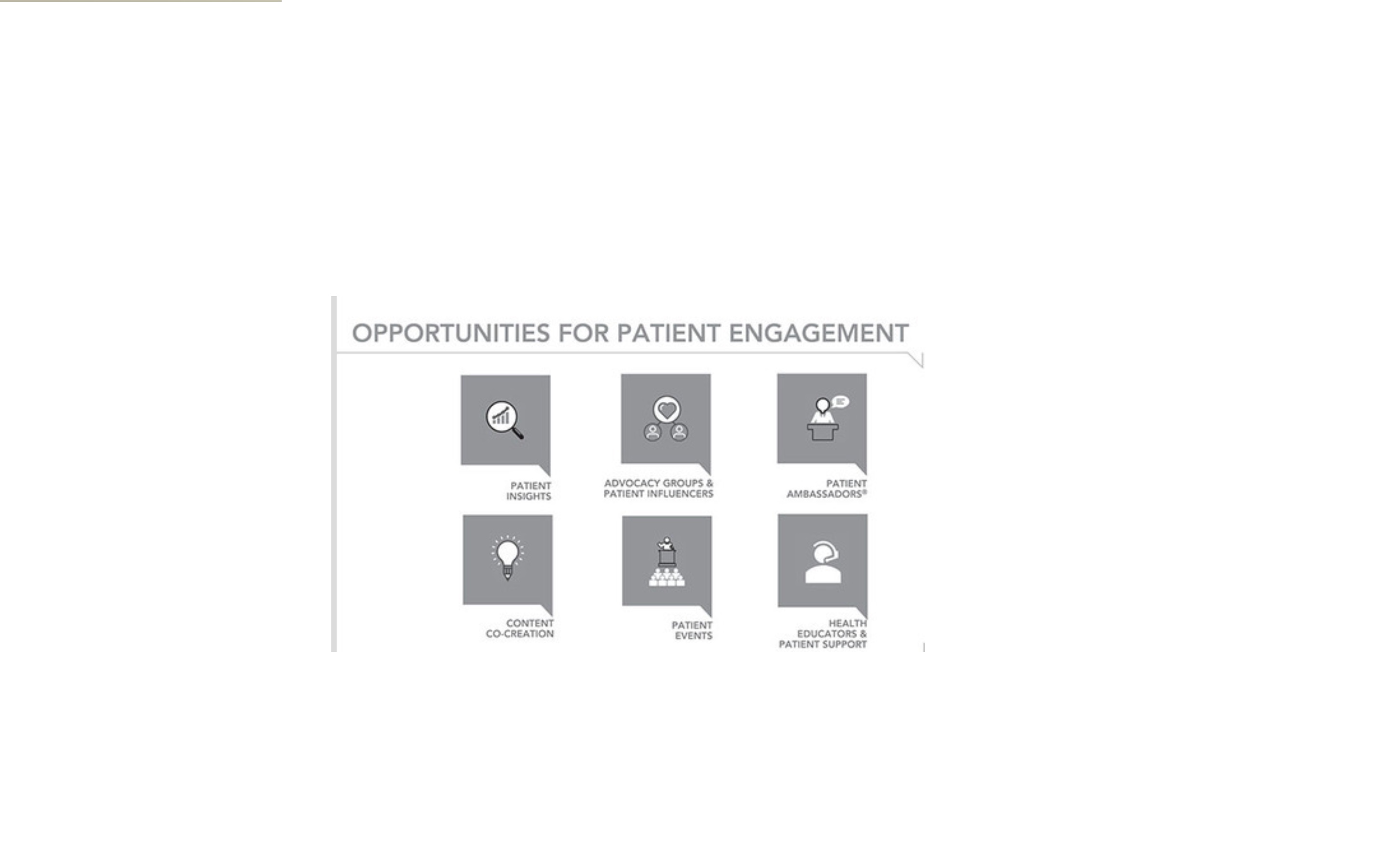
Snow Companies also offers a unique Patient Ambassador program. The company sources, develops, trains, and manages Patient Ambassadors, who are people with a medical condition who have undergone legal, regulatory, and storytelling training. They help provide a human face to the disease conditions and the brands they represent. Patient Ambassadors help raise awareness of treatment options and educate others about therapy choices by sharing their personal stories. In this role, they become a resource for other patients with the same condition under the guidelines established by the FDA. This powerful and persuasive personal communication has proven to be a success and has helped Snow Companies become a leading marketing force in the pharmaceutical and biotech industry.
Snow Companies employs a variety of direct marketing strategies in the execution of tactics to support clients, including direct mail, outbound and inbound telephone marketing, e-mail marketing, Internet marketing, and social networking to reach target audiences. Patient Ambassadors are used in a mixture of online features, including webisodes, You Tube videos, and features on sponsored websites. Web initiatives, as a percentage of a brands tactical mix, continue to rise due to decreasing brand budgets. This means that there is increasing scrutiny for effectively measuring marketing spending and the desire to target specific patient segments.
To date, Snow Companies has conducted Patient Ambassador programs in 25 countries, trained tens of thousands of Patient Ambassadors, and connected more than eight million people with their stories. Moreover, it is currently active in more than 150 disease categories and has 75 industry partners. Most importantly, the program gives patients and care partners the knowledge and motivation they need to successfully manage and live with their respective health conditions. Telling and listening to stories is so powerful and has the potential to alter behavior and improve health. Thats quite an impact! Further, although face-to-face interactions have the greatest impact on behavior, patients are increasingly leveraging the Internet, and specifically social media, for health topics such as a specific disease or treatment.
Despite successfully using Patient Ambassadors in content online, such as posting approved material on a program Facebook site or listing Local Patient Outreach Programs (LPOPs) for their social network to attend, there are still restrictions. Although DTC marketing has moved online and will remain there for the foreseeable future, social media continues to be an area where communications are limited or tentative. Pharmaceutical companies producing online video content have disabled You Tube commenting, for example, due to HIPAA concerns over the friends and subscribers functions, which might reveal the identity (and diagnosis) of those parties. Depending on the content of a channels posted videos, that is, branded versus unbranded information, additional restrictions may be made related to disclaimers, share functions, and friend/subscriber options.
Besides patient privacy issues, another major concern for the pharma and biotech industry is the reporting of adverse events (AEs), or side-effects, via the Internet. Members of the industry are required to report AEs within 24 hours of receiving information that fits four criteria: an identifiable reporter, patient, drug name, and adverse event. These requirements increase the burden on any marketing initiatives from a financial and resource perspective, as constant surveillance would be mandatory.
The subsequent surveillance and anticipated results must be weighed to determine if this is an avenue that is positive for the brand. Additionally, Facebook has made screening responses more challenging by lifting the ability to prescreen comments in 2011. This decision makes it even more challenging for pharmaceutical companies to engage with their consumers directly, due to the increased risks related to other regulatory guidelines. The FDA has been studying social media for several years, and in 2014 it made available draft guidelines for the industry entitled Internet/Social Media Platforms with Character Space Limitations: Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices. The agency continues to work in this ever-evolving digital landscape, with more regulations likely to come in the future.
In any case, whether a brand chooses to engage in digital and social mediums or not, in order to remain authentic, real patients should be a major part of the brands tactics. The more involved these brand ambassadors are, the stronger the messages, brand resonance, and subsequent marketing results. Snow continues to look for new, yet safe, approaches to social media and pharma marketing, and will continue to propose and develop new ways to leverage the conversations patients are having online.
Because of the highly regulated nature of the industry, all of the companys activities come under tough scrutiny from regulatory, legal, and medical reviewers who closely monitor all of these activities to ensure compliance. One of the most effective methods used by Snow Companies is live events, which are high-touch and very resonant with the people who attend. LPOPs are targeted educational symposia for patients, caregivers, family members, and friends to learn about a specific condition and possible treatments for that condition. Snow uses direct marketing tactics to promote LPOPs and find people who are interested in learning more about their condition and interacting with others living with it.
At these events, a Patient Ambassador shares their personal storynot just their trials and tribulations of living with a chronic medical condition, but also the inspiring, the funny, and the elevating parts of their journey. They also share their philosophy of taking charge of their health to inspire others, as well as sharing tips and advice for their specific condition. Disease and treatment information is presented by a healthcare professional. The program attendees can also meet others living with the same condition or opt in for relationship marketing programs for more information. Through these programs, Snow is able to amplify brand messages, such as treatment compliance, not settling, and being proactive with healthcare providers, while still remaining compliant with the industry guidelines.
Conclusion
Snow Companies is an excellent example of an organization that has found a way to be highly successful by effectively employing myriad direct and interactive marketing strategies and tactics within the constraints of a strict regulatory environment.
Case Discussion Questions
- Discuss the factors contributing to the popularity of direct-to-customer (DTC) marketing in the pharmaceutical industry. Explain the advantages and disadvantages of direct-to-customer marketing of prescription drugs to patients, physicians, and pharmaceutical companies.
- Describe the number of reviews a pharmaceutical product has to go through before it is marketed to the public. Explain how each review committee is made up and the rationale behind it.
- Discuss Snow Companies and the Patient Ambassador program developed by Brenda Snow. Who are the Patient Ambassadors and what role(s) do they play in the marketing of pharmaceutical and biotech products? Explain the communication techniques that they use.
- Check out the most recent FDA policies on social media usage by pharmaceutical marketers to reveal the latest legal regulations that are in place to police the marketing activities of this highly regulated industry. What future directions do you think the FDA will take?
- Why are pharmaceutical and biotech marketers wary about using large-scale social networking to promote their products? Explain the advantages and disadvantages of social networking in marketing pharmaceutical and biotech products.
OPPORTUNITIES FOR PATIENT ENGAGEMENT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


