Question: Do the above problem with the below steps: Problem 5 The system diphenylhexane-docosane-furfural is representative of more complex systems encountered in the solvent refining of
Do the above problem with the below steps:
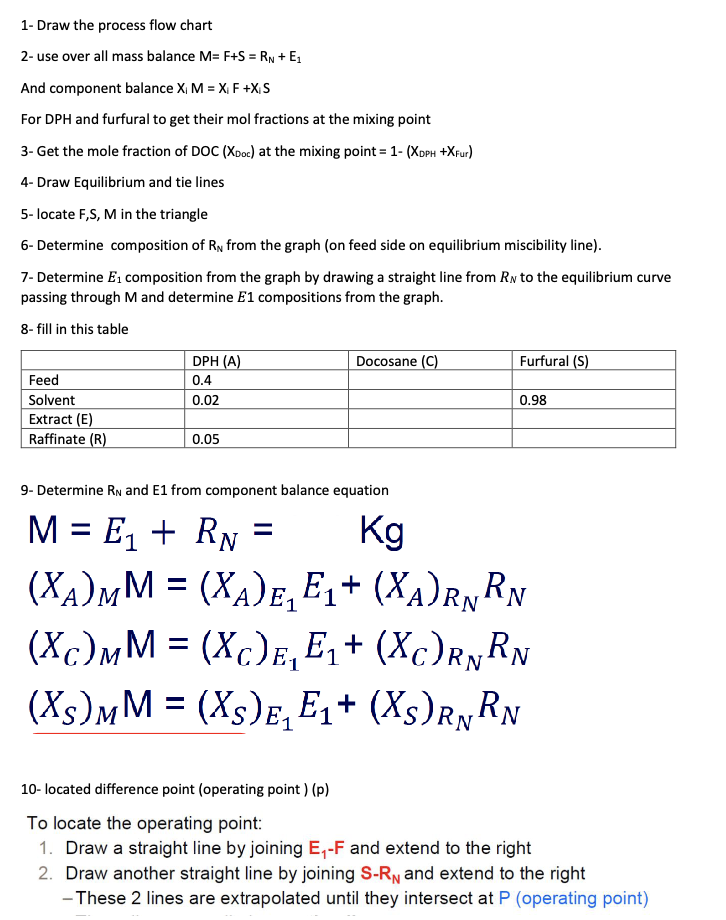
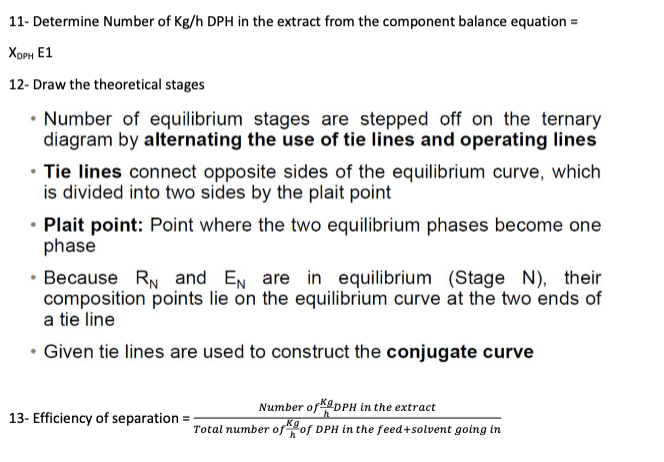
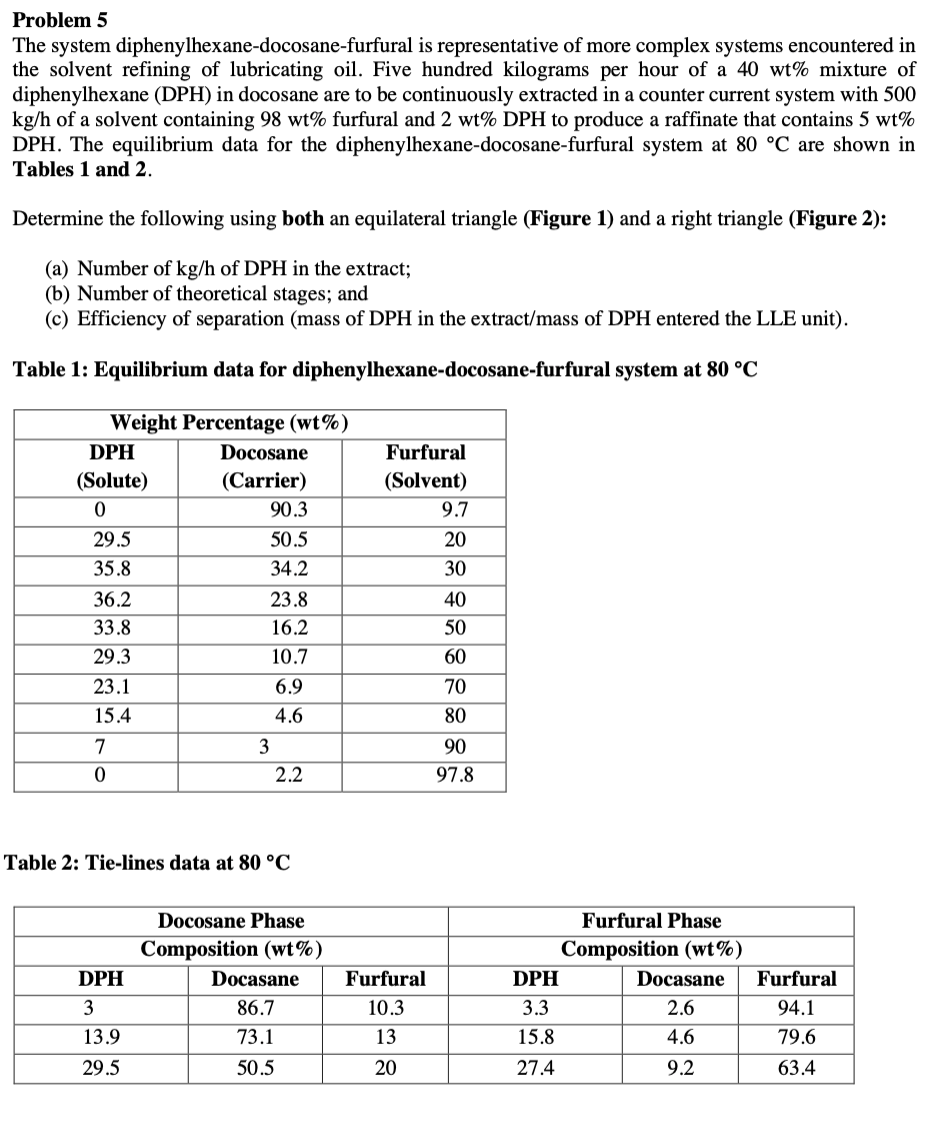
Problem 5 The system diphenylhexane-docosane-furfural is representative of more complex systems encountered in the solvent refining of lubricating oil. Five hundred kilograms per hour of a 40wt% mixture of diphenylhexane (DPH) in docosane are to be continuously extracted in a counter current system with 500 kg/h of a solvent containing 98wt% furfural and 2wt%DPH to produce a raffinate that contains 5wt% DPH. The equilibrium data for the diphenylhexane-docosane-furfural system at 80C are shown in Tables 1 and 2. Determine the following using both an equilateral triangle (Figure 1) and a right triangle (Figure 2): (a) Number of kg/h of DPH in the extract; (b) Number of theoretical stages; and (c) Efficiency of separation (mass of DPH in the extract/mass of DPH entered the LLE unit). Table 1: Equilibrium data for diphenylhexane-docosane-furfural system at 80C Table 2: Tie-lines data at 80C Figure 1: Equilateral triangle template for LLE 1- Draw the process flow chart 2- use over all mass balance M=F+S=RN+E1 And component balance XiM=XiF+XiS For DPH and furfural to get their mol fractions at the mixing point 3- Get the mole fraction of DOC(XDoc) at the mixing point =1(XDPH+Xfur) 4- Draw Equilibrium and tie lines 5- locate F,S,M in the triangle 6- Determine composition of RN from the graph (on feed side on equilibrium miscibility line). 7- Determine E1 composition from the graph by drawing a straight line from RN to the equilibrium curve passing through M and determine E1 compositions from the graph. 8- fill in this table 9- Determine RN and E1 from component balance equation M=E1+RN=Kg(XA)MM=(XA)E1E1+(XA)RNRN(XC)MM=(XC)E1E1+(XC)RNRN(XS)MM=(XS)E1E1+(XS)RNRN 10- located difference point (operating point ) (p) To locate the operating point: 1. Draw a straight line by joining E1F and extend to the right 2. Draw another straight line by joining SRN and extend to the right - These 2 lines are extrapolated until they intersect at P (operating point) XDPHE1 12- Draw the theoretical stages - Number of equilibrium stages are stepped off on the ternary diagram by alternating the use of tie lines and operating lines - Tie lines connect opposite sides of the equilibrium curve, which is divided into two sides by the plait point - Plait point: Point where the two equilibrium phases become one phase - Because RN and EN are in equilibrium (Stage N ), their composition points lie on the equilibrium curve at the two ends of a tie line - Given tie lines are used to construct the conjugate curve 13- Efficiency of separation =TotalnumberofhKgofDPHinthefeed+solventgoinginNumberofhKgDPHintheextract Problem 5 The system diphenylhexane-docosane-furfural is representative of more complex systems encountered in the solvent refining of lubricating oil. Five hundred kilograms per hour of a 40wt% mixture of diphenylhexane (DPH) in docosane are to be continuously extracted in a counter current system with 500 kg/h of a solvent containing 98wt% furfural and 2wt%DPH to produce a raffinate that contains 5wt% DPH. The equilibrium data for the diphenylhexane-docosane-furfural system at 80C are shown in Tables 1 and 2. Determine the following using both an equilateral triangle (Figure 1) and a right triangle (Figure 2): (a) Number of kg/h of DPH in the extract; (b) Number of theoretical stages; and (c) Efficiency of separation (mass of DPH in the extract/mass of DPH entered the LLE unit). Table 1: Equilibrium data for diphenylhexane-docosane-furfural system at 80C Table 2: Tie-lines data at 80C Figure 1: Equilateral triangle template for LLE 1- Draw the process flow chart 2- use over all mass balance M=F+S=RN+E1 And component balance XiM=XiF+XiS For DPH and furfural to get their mol fractions at the mixing point 3- Get the mole fraction of DOC(XDoc) at the mixing point =1(XDPH+Xfur) 4- Draw Equilibrium and tie lines 5- locate F,S,M in the triangle 6- Determine composition of RN from the graph (on feed side on equilibrium miscibility line). 7- Determine E1 composition from the graph by drawing a straight line from RN to the equilibrium curve passing through M and determine E1 compositions from the graph. 8- fill in this table 9- Determine RN and E1 from component balance equation M=E1+RN=Kg(XA)MM=(XA)E1E1+(XA)RNRN(XC)MM=(XC)E1E1+(XC)RNRN(XS)MM=(XS)E1E1+(XS)RNRN 10- located difference point (operating point ) (p) To locate the operating point: 1. Draw a straight line by joining E1F and extend to the right 2. Draw another straight line by joining SRN and extend to the right - These 2 lines are extrapolated until they intersect at P (operating point) XDPHE1 12- Draw the theoretical stages - Number of equilibrium stages are stepped off on the ternary diagram by alternating the use of tie lines and operating lines - Tie lines connect opposite sides of the equilibrium curve, which is divided into two sides by the plait point - Plait point: Point where the two equilibrium phases become one phase - Because RN and EN are in equilibrium (Stage N ), their composition points lie on the equilibrium curve at the two ends of a tie line - Given tie lines are used to construct the conjugate curve 13- Efficiency of separation =TotalnumberofhKgofDPHinthefeed+solventgoinginNumberofhKgDPHintheextract
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


