Question: don't copy I will dislike Methane and oxygen react in the presence of a catalyst to form formaldehyde. In a parallel reaction, methane is oxidized
don't copy I will dislike
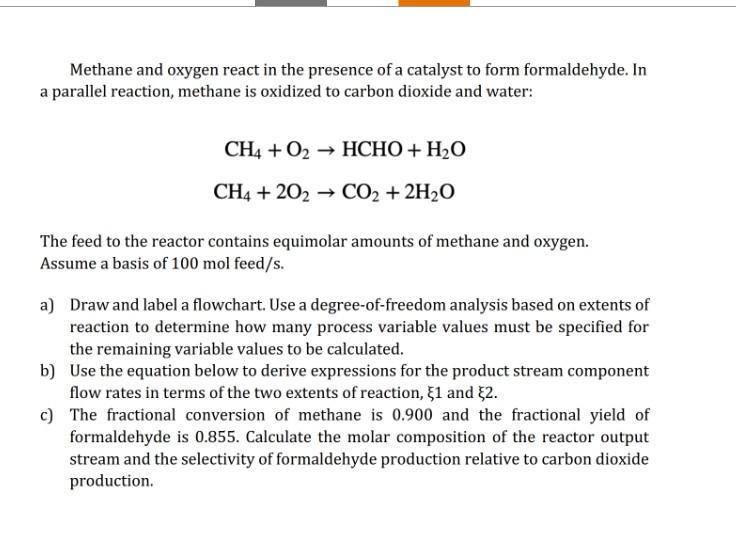
Methane and oxygen react in the presence of a catalyst to form formaldehyde. In a parallel reaction, methane is oxidized to carbon dioxide and water: CH4+O2CH4+2O2HCHO+H2OCO2+2H2O The feed to the reactor contains equimolar amounts of methane and oxygen. Assume a basis of 100mol feed/s. a) Draw and label a flowchart. Use a degree-of-freedom analysis based on extents of reaction to determine how many process variable values must be specified for the remaining variable values to be calculated. b) Use the equation below to derive expressions for the product stream component flow rates in terms of the two extents of reaction, 1 and 2. c) The fractional conversion of methane is 0.900 and the fractional yield of formaldehyde is 0.855 . Calculate the molar composition of the reactor output stream and the selectivity of formaldehyde production relative to carbon dioxide production
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


