Question: dont know what i did wrong A reaction AB has the following time dependence for the concentration of [A] vs time. For t=(0s,5s,10s,15s,25s) the concentration
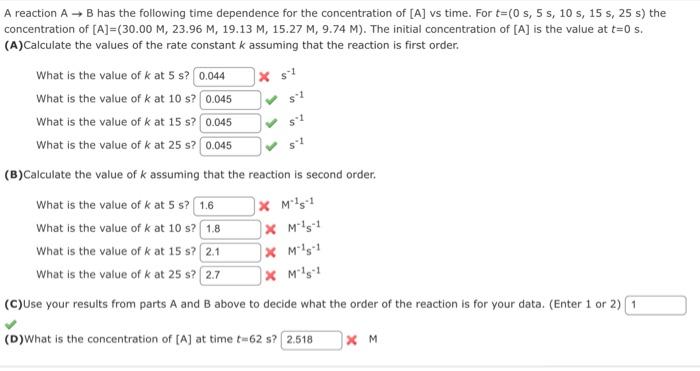
A reaction AB has the following time dependence for the concentration of [A] vs time. For t=(0s,5s,10s,15s,25s) the concentration of [A]=(30.00M,23.96M,19.13M,15.27M,9.74M). The initial concentration of [A] is the value at t=0s. (A)Calculate the values of the rate constant k assuming that the reaction is first order. What is the value of k at 5s ? * s1 What is the value of k at 10s ? s1 What is the value of k at 15s ? s1 What is the value of k at 25s ? s1 (B) Calculate the value of k assuming that the reaction is second order. What is the value of k at 5s ? M1s1 What is the value of k at 10s ? M1s1 What is the value of k at 15s ? M1s1 What is the value of k at 25s ? M1s1 (C) Use your results from parts A and B above to decide what the order of the reaction is for your data. (Enter 1 or 2) (D) What is the concentration of [A] at time t=62s ? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


