Question: dont skip the steps please 3. (20 pts) Consider that an elementary, irreversible, liquid-phase reaction: A+B2C is carried out in a continuous stirred-tank reactor (CSTR).
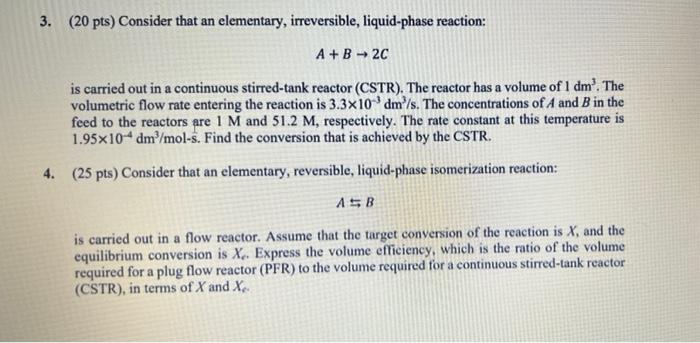
3. (20 pts) Consider that an elementary, irreversible, liquid-phase reaction: A+B2C is carried out in a continuous stirred-tank reactor (CSTR). The reactor has a volume of 1 dm'. The volumetric flow rate entering the reaction is 3.3x10-dm/s. The concentrations of A and B in the feed to the reactors are 1 M and 51.2 M, respectively. The rate constant at this temperature is 1.95x10-4 dm /mol-s. Find the conversion that is achieved by the CSTR. 4. (25 pts) Consider that an elementary, reversible, liquid-phase isomerization reaction: ASB is carried out in a flow reactor. Assume that the target conversion of the reaction is X, and the equilibrium conversion is X.. Express the volume efficiency, which is the ratio of the volume required for a plug flow reactor (PFR) to the volume required for a continuous stirred-tank reactor (CSTR), in terms of X and X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


