Question: Draw Lewis structure(s) showing all possible equivalent resonance forms for the nitryl chloride molecule (NOCI). Draw one structure per sketcher box, and separate any


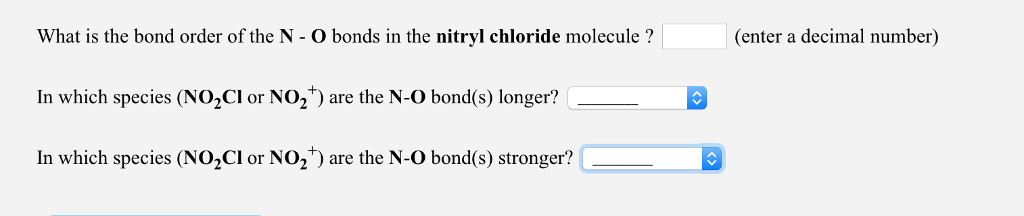
Draw Lewis structure(s) showing all possible equivalent resonance forms for the nitryl chloride molecule (NOCI). Draw one structure per sketcher box, and separate any added sketcher boxes with the bonds to oxygen unless they are needed for the central atom to obey the octet rule. NOCl : symbol. Do NOT show any ion charges in your drawings. Do not draw double Draw Lewis structure(s) showing all possible equivalent resonance forms for the nitronium ion (NO*). Draw one structure per sketcher box, and separate any added sketcher boxes with the bonds to oxygen unless they are needed for the central atom to obey the octet rule. NO*: symbol. Do NOT show any ion charges in your drawings. Do not draw double What is the bond order of the N - O bonds in the nitryl chloride molecule ? In which species (NOCl or NO) are the N-O bond(s) longer? In which species (NOCl or NO) are the N-O bond(s) stronger? (enter a decimal number)
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


