Question: Draw out the compounds as tetrahedral and square planar. Using this information make a prediction: assign whether Ni(PPh3)2Cl2 and Ni(PCy3)2Cl2 are predicted to be tetrahedral
Draw out the compounds as tetrahedral and square planar. Using this information make a prediction: assign whether Ni(PPh3)2Cl2 and Ni(PCy3)2Cl2 are predicted to be tetrahedral or square planar. Using Hunds Rule, place the electrons into the diagrams. Do you predict either molecule to be paramagnetic (have unpaired spins)? If so, which one(s)? 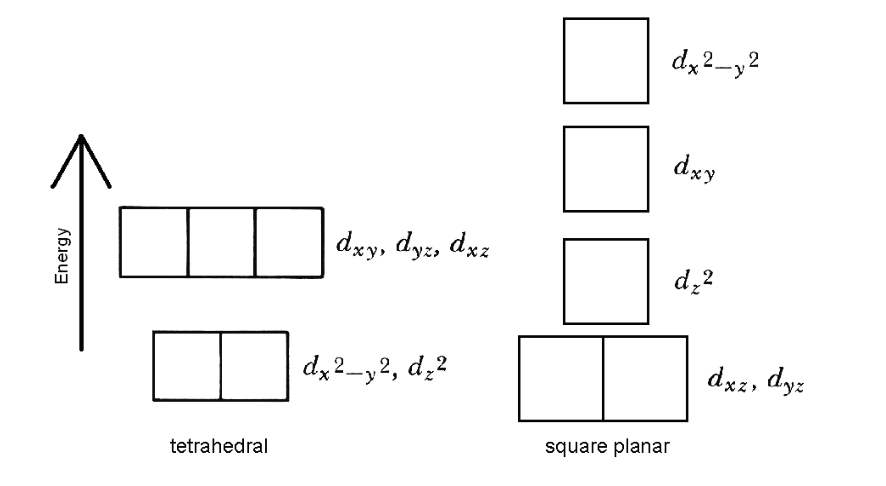
dx_2 dxy Energy dxy, dyz dxz d22 dx2-,2, d_2 dxz, dyz tetrahedral square planar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


