Question: due soon! need help Current Attempt in Progress A combustion chamber is used to burn a fuel mixture consisting of methane and ethane. The fuel

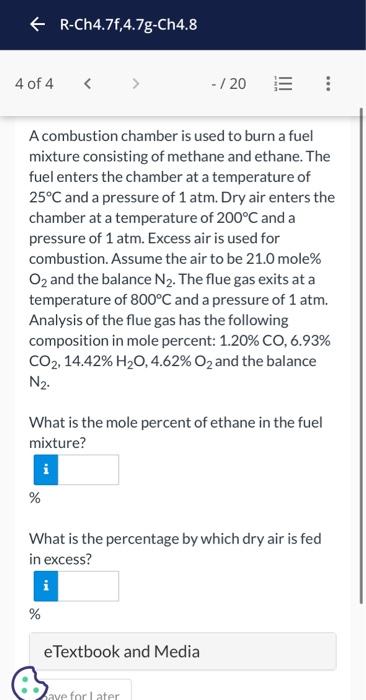
Current Attempt in Progress A combustion chamber is used to burn a fuel mixture consisting of methane and ethane. The fuel enters the chamber at a temperature of 25C and a pressure of 1atm. Dry air enters the chamber at a temperature of 200C and a pressure of 1atm. Excess air is used for combustion. Assume the air to be 21.0 mole\% O2 and the balance N2. The flue gas exits at a temperature of 800C and a pressure of 1atm. Analysis of the flue gas has the following composition in mole percent: 1.20%CO,6.93% CO2,14.42%H2O,4.62%O2 and the balance N2. What is the mole percent of ethane in the fuel mixture? A combustion chamber is used to burn a fuel mixture consisting of methane and ethane. The fuel enters the chamber at a temperature of 25C and a pressure of 1atm. Dry air enters the chamber at a temperature of 200C and a pressure of 1atm. Excess air is used for combustion. Assume the air to be 21.0mole% O2 and the balance N2. The flue gas exits at a temperature of 800C and a pressure of 1atm. Analysis of the flue gas has the following composition in mole percent: 1.20%CO,6.93% CO2,14.42%H2O,4.62%O2 and the balance N2. What is the mole percent of ethane in the fuel mixture? % What is the percentage by which dry air is fed in excess? %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


