Question: During my first Environmental Chemistry class, the instructor indicated the partial pressure of CO2 in the earth's atmosphere was PCO2=103.439atm. This information was taken from
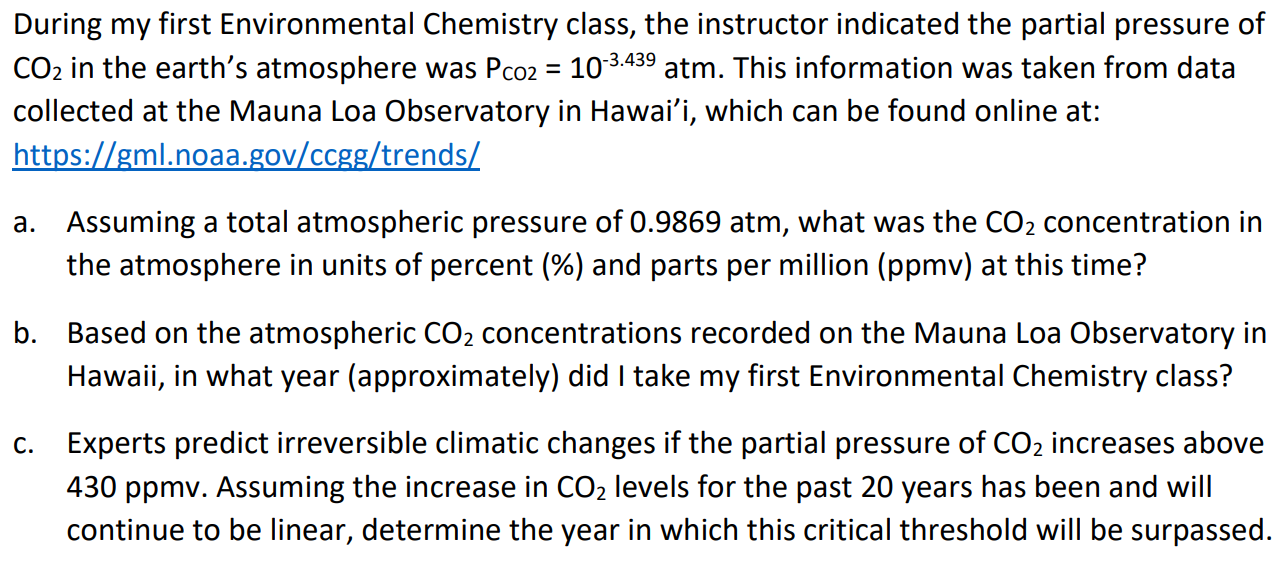
During my first Environmental Chemistry class, the instructor indicated the partial pressure of CO2 in the earth's atmosphere was PCO2=103.439atm. This information was taken from data collected at the Mauna Loa Observatory in Hawai'i, which can be found online at: https://gml.noaa.gov/ccgg/trends/ a. Assuming a total atmospheric pressure of 0.9869 atm, what was the CO2 concentration in the atmosphere in units of percent (\%) and parts per million ( ppmv) at this time? b. Based on the atmospheric CO2 concentrations recorded on the Mauna Loa Observatory in Hawaii, in what year (approximately) did I take my first Environmental Chemistry class? c. Experts predict irreversible climatic changes if the partial pressure of CO2 increases above 430 ppmv. Assuming the increase in CO2 levels for the past 20 years has been and will continue to be linear, determine the year in which this critical threshold will be surpassed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


