Question: During the Contact Process SO3 is produced from SO2 in a reversible reaction: 2SO2(g)+O2(g)2SO3(g) Write an expression for the equilibrium constant, Kc, and derive its
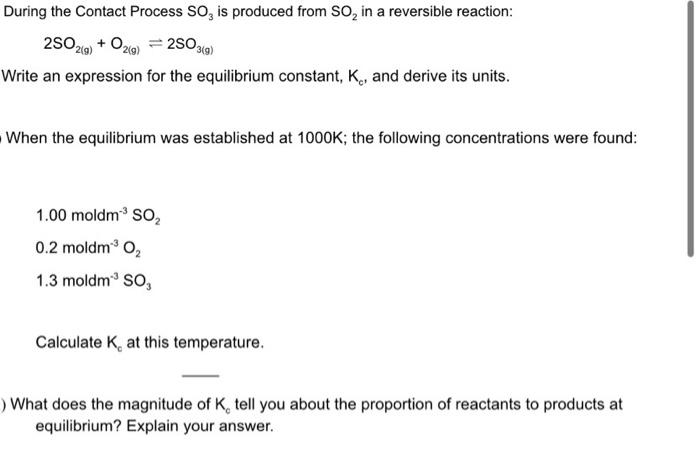
During the Contact Process SO3 is produced from SO2 in a reversible reaction: 2SO2(g)+O2(g)2SO3(g) Write an expression for the equilibrium constant, Kc, and derive its units. When the equilibrium was established at 1000K; the following concentrations were found: 1.00moldm3SO20.2moldm3O21.3moldm3SO3 Calculate Kc at this temperature. What does the magnitude of Kc tell you about the proportion of reactants to products at equilibrium? Explain your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


