Question: During vinegar analysis, a 2.0 mL vinegar sample (d =1.0 g/mL, %(w/w) CH3COOH= 5.5%, (molar mass 60 g/mol) was titrated with 0.2M NaOH, If the
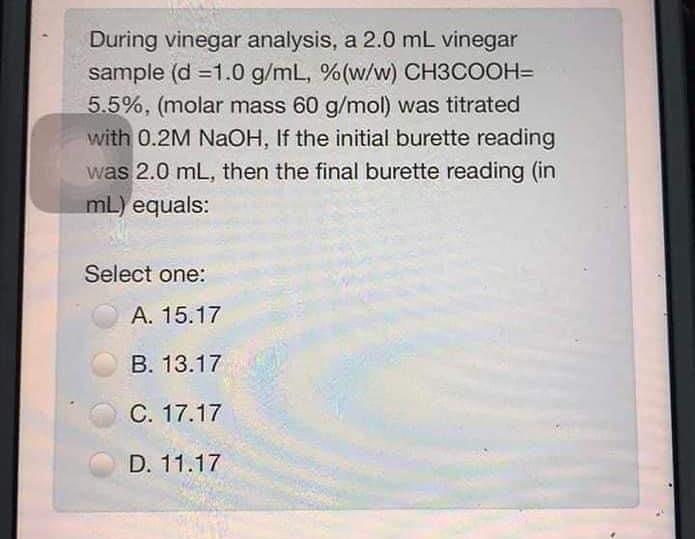
During vinegar analysis, a 2.0 mL vinegar sample (d =1.0 g/mL, %(w/w) CH3COOH= 5.5%, (molar mass 60 g/mol) was titrated with 0.2M NaOH, If the initial burette reading was 2.0 mL, then the final burette reading in mL) equals: Select one: A. 15.17 B. 13.17 C. 17.17 D. 11.17
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


