Question: E 4 . 2 . 5 The Redlich - Kwong model for fluids has a P v T equation of state P = R T
E The RedlichKwong model for fluids has a equation of state
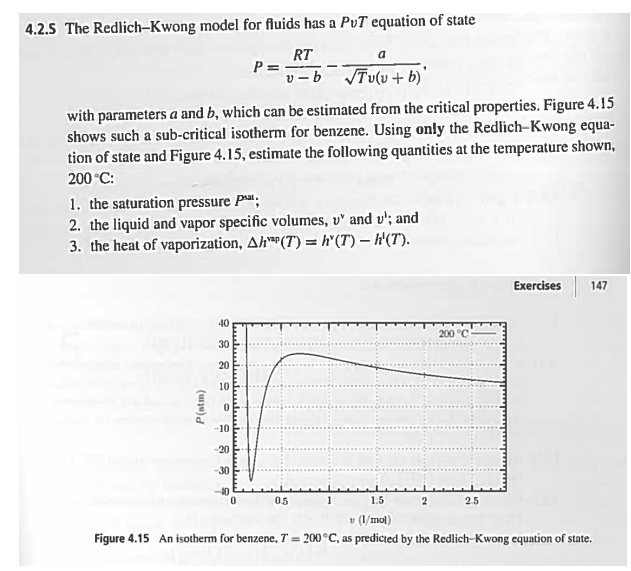
with parameters a and which can be estimated from the critical properties. Figure
shows such a subcritical isotherm for benzene. Using only the RedichKwong equa
tion of state and Figure estimate the following quantities at the temperature shown,
:
the saturation pressure ;
the liquid and vapor specific volumes, and ; and
the heat of vaporization,
Figure An isotherm for benzene, as predicted by the RedlichKwong equation of state.quation of state and Figure estimate the following quantities at the temperature shown,
:
a find the saturation pressure ;
b find the liquid and vapor specific volumes, and ; and
c find the heat of vaporization,
use given Figure An isotherm for benzene, as predicted by the RedlichKwong equation of state.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


