Question: e) Construct the potential-pH diagram for zinc using the following reactions and assuming the activity of all dissolved substances is 10-6.| a. Zn= Zn2+ +
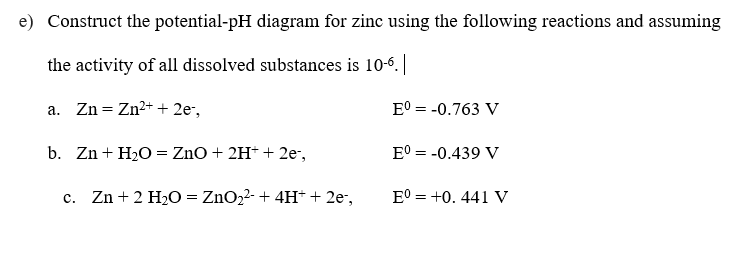
e) Construct the potential-pH diagram for zinc using the following reactions and assuming the activity of all dissolved substances is 10-6.| a. Zn= Zn2+ + 2e-, E = -0.763 V b. Zn + H2O = ZnO + 2H+ + 2e, E = -0.439 V = c. Zn+ 2 H2O = ZnO22- + 4H+ + 2e-, E = +0.441 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


