Question: e) what would be the activation energy for the reverse reaction? Isider the reaction: A+BC. The diagram below represents the energy profile for this reaction.
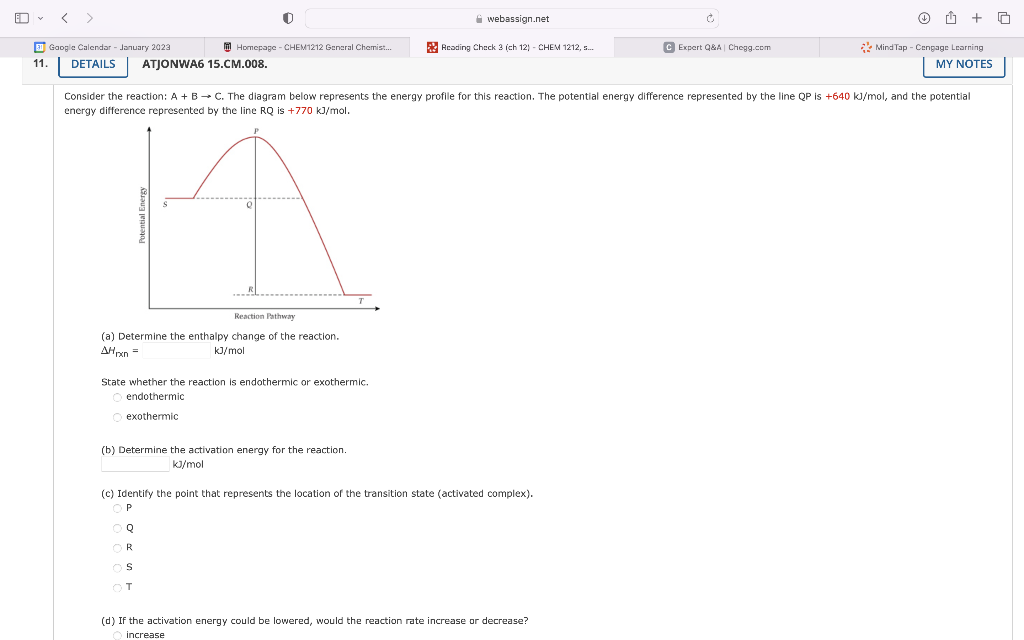
e) what would be the activation energy for the reverse reaction?
Isider the reaction: A+BC. The diagram below represents the energy profile for this reaction. The potential energy difference represented by the line QP is +640 kJ/mol, and the potential irgy difference represented by the line RQ is +770kJ/mol. (a) Determine the enthalpy change of the reaction. H1rn= kJ/mol State whether the reaction is endothermic or exothermic. endothermic exothermic (b) Determine the activation energy for the reaction. kJ/mol (c) Identify the point that represents the location of the transition state (activated complex). P Q R s T (d) If the activation energy could be lowered, would the reaction rate increase or decrease? increase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


