Question: E=4oe2RoZ1Z2 The first term is a constant for any solid so the electrostatic potential energy term for a given ceramic solid is proportional to z1
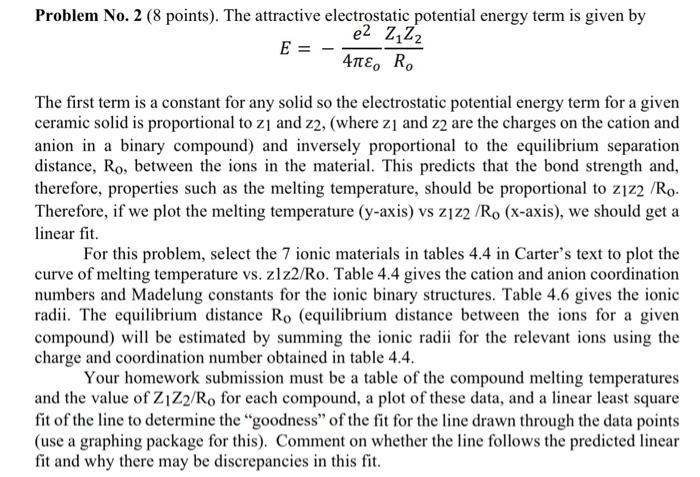
E=4oe2RoZ1Z2 The first term is a constant for any solid so the electrostatic potential energy term for a given ceramic solid is proportional to z1 and z2, (where z1 and z2 are the charges on the cation and anion in a binary compound) and inversely proportional to the equilibrium separation distance, Ro, between the ions in the material. This predicts that the bond strength and, therefore, properties such as the melting temperature, should be proportional to z1z2/R0. Therefore, if we plot the melting temperature ( y-axis) vs z1z2/R0 (x-axis), we should get a linear fit. For this problem, select the 7 ionic materials in tables 4.4 in Carter's text to plot the curve of melting temperature vs. z1z2/Ro. Table 4.4 gives the cation and anion coordination numbers and Madelung constants for the ionic binary structures. Table 4.6 gives the ionic radii. The equilibrium distance R0 (equilibrium distance between the ions for a given compound) will be estimated by summing the ionic radii for the relevant ions using the charge and coordination number obtained in table 4.4. Your homework submission must be a table of the compound melting temperatures and the value of Z1Z2/Ro for each compound, a plot of these data, and a linear least square fit of the line to determine the "goodness" of the fit for the line drawn through the data points (use a graphing package for this). Comment on whether the line follows the predicted linear fit and why there may be discrepancies in this fit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


