Question: Each value represents a different aqueous solution at 25C. Classify each solution as acidic, basic, or neutral. Answer Bank [OH]=9.5102 pH=9.69pOH=7.00pH=3.36 [H+]=4.0105 [H+]=1.0107 [H+]=1.31011 pOH=5.97
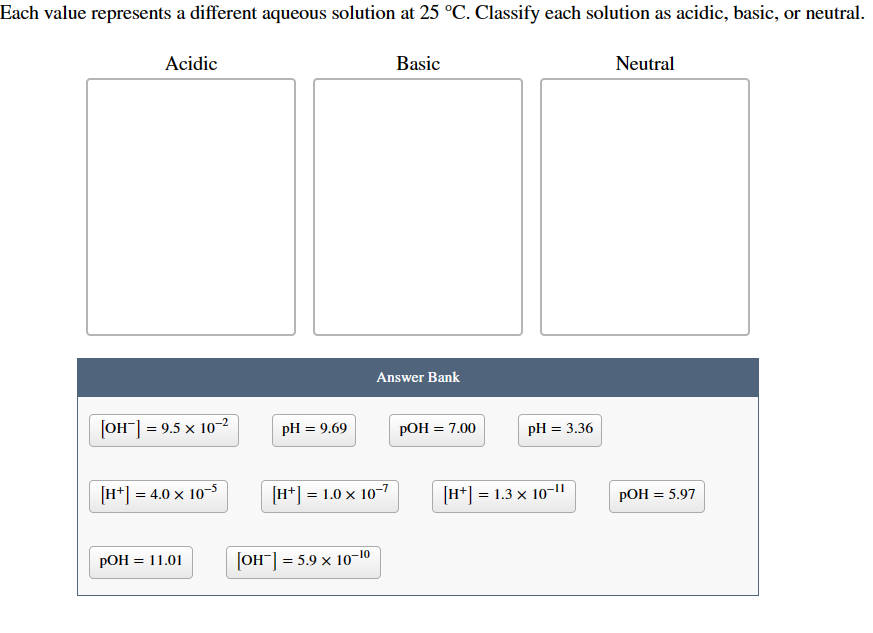
Each value represents a different aqueous solution at 25C. Classify each solution as acidic, basic, or neutral. Answer Bank [OH]=9.5102 pH=9.69pOH=7.00pH=3.36 [H+]=4.0105 [H+]=1.0107 [H+]=1.31011 pOH=5.97 pOH=11.01 [OH]=5.91010
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


