Question: Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular weight (gm/mole). Retention Time and Molecular Weight
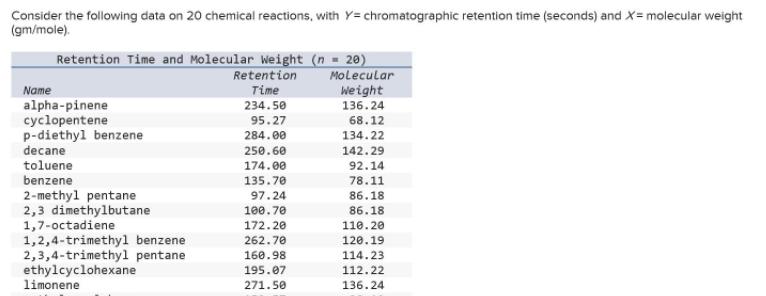
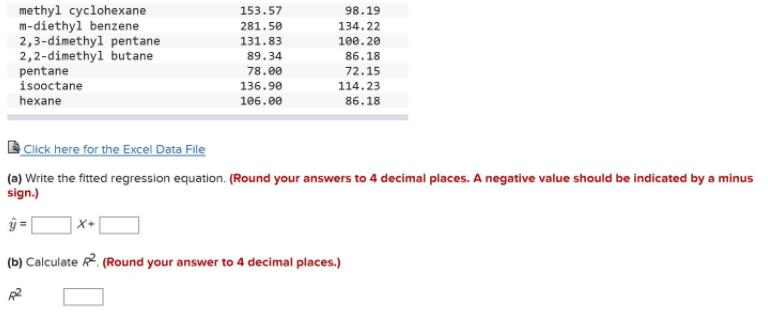
Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular weight (gm/mole). Retention Time and Molecular Weight (n = 20) Molecular Weight Retention Name Time alpha-pinene cyclopentene p-diethyl benzene decane toluene benzene 234.50 136.24 95.27 68.12 284.00 134.22 250.60 142.29 174.00 92.14 135.70 78.11 2-methyl pentane 2,3 dimethylbutane 1,7-octadiene 1,2,4-trimethyl benzene 2,3,4-trimethyl pentane ethylcyclohexane limonene 97.24 86.18 100.70 86.18 172.20 110.20 262.70 120.19 114.23 112.22 160.98 195.07 271.50 136.24 methyl cyclohexane m-diethyl benzene 2,3-dimethyl pentane 2,2-dimethyl butane pentane isooctane 153.57 98.19 281.50 134.22 131.83 100.20 89.34 86.18 78.00 72.15 136.90 114.23 hexane 106.00 86.18 Click here for the Excel Data File (a) Write the fitted regression equation. (Round your answers to 4 decimal places. A negative value should be indicated by a minus sign.) (b) Calculate R. (Round your answer to 4 decimal places.)
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
To address the problem well perform a linear regression analysis using the given data Wel... View full answer

Get step-by-step solutions from verified subject matter experts


